Rapid SARS-CoV-2 Detection Using the Lucira™ Check It COVID-19 Test Kit
- PMID: 36010227
- PMCID: PMC9406928
- DOI: 10.3390/diagnostics12081877
Rapid SARS-CoV-2 Detection Using the Lucira™ Check It COVID-19 Test Kit
Abstract
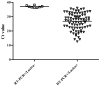
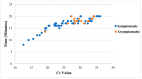
The need for the early identification of SARS-CoV-2 has let to a quest for reliable tests that meet the standards of polymerase chain reaction (PCR) tests, on the one hand, and are low-cost, easy-to-use, and fast, on the other hand. One such test is the Lucira™ Check It COVID-19 Test kit (“Lucira”) (Lucira Health, Inc., Emeryville, CA, USA), which utilizes real-time loop-mediated isothermal amplification technology, developed for at-home use. This study evaluated the clinical sensitivity and specificity of Lucira in identifying the virus in 190 nasopharyngeal samples collected between January and October 2021. Each sample was also subjected to RT-PCR. All negative RT-PCR results were paralleled by a negative Lucira result. Out of 90 participants who had a positive RT-PCR result, 82 (91.1%) tested positive by Lucira. Among the 72 symptomatic participants, 67 (93%) tested positive by Lucira. All samples with a positive RT-PCR result with a threshold cycle (Ct) > 36, yielded a negative Lucira result. In addition, a significant positive correlation was found between Ct and time-to-positivity with Lucira (R = 0.8612, p < 0.0001). The implementation of such a portable and affordable assay may aid in breaking the COVID-19 transmission chain.
Keywords: COVID-19; LAMP; Lucira; kit performance; rapid diagnosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- WHO Coronavirus (COVID-19) Dashboard. [(accessed on 1 June 2022)]. Available online: https://covid19.who.int.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

