Contrast-Enhanced Mammography versus Breast Magnetic Resonance Imaging: A Systematic Review and Meta-Analysis
- PMID: 36010240
- PMCID: PMC9406751
- DOI: 10.3390/diagnostics12081890
Contrast-Enhanced Mammography versus Breast Magnetic Resonance Imaging: A Systematic Review and Meta-Analysis
Abstract
Background: Contrast-enhanced mammography (CEM) and contrast-enhanced magnetic resonance imaging (CE-MRI) are commonly used in the screening of breast cancer. The present systematic review aimed to summarize, critically analyse, and meta-analyse the available evidence regarding the role of CE-MRI and CEM in the early detection, diagnosis, and preoperative assessment of breast cancer.
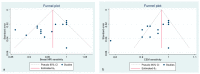
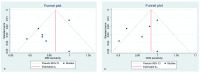
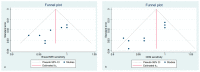
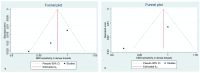
Methods: The search was performed on PubMed, Google Scholar, and Web of Science on 28 July 2021 using the following terms "breast cancer", "preoperative staging", "contrast-enhanced mammography", "contrast-enhanced spectral mammography", "contrast enhanced digital mammography", "contrast-enhanced breast magnetic resonance imaging" "CEM", "CESM", "CEDM", and "CE-MRI". We selected only those papers comparing the clinical efficacy of CEM and CE-MRI. The study quality was assessed using the QUADAS-2 criteria. The pooled sensitivities and specificity of CEM and CE-MRI were computed using a random-effects model directly from the STATA "metaprop" command. The between-study statistical heterogeneity was tested (I2-statistics).
Results: Nineteen studies were selected for this systematic review. Fifteen studies (1315 patients) were included in the metanalysis. Both CEM and CE-MRI detect breast lesions with a high sensitivity, without a significant difference in performance (97% and 96%, respectively).
Conclusions: Our findings confirm the potential of CEM as a supplemental screening imaging modality, even for intermediate-risk women, including females with dense breasts and a history of breast cancer.
Keywords: breast cancer; contrast-enhanced breast magnetic resonance imaging; contrast-enhanced mammography; screening.
Conflict of interest statement
Chiti reports a fellowship grant from Sanofi, personal fees from AAA, Blue Earth Diagnostics and General Electric Healthcare, outside the submitted work. The other authors do not report any conflict of interest.
Figures







Similar articles
-
Contrast-enhanced Mammography versus Contrast-enhanced Breast MRI: A Systematic Review and Meta-Analysis.Radiology. 2022 Oct;305(1):94-103. doi: 10.1148/radiol.212530. Epub 2022 Jun 7. Radiology. 2022. PMID: 36154284
-
Contrast-Enhanced Digital Breast Tomosynthesis Compared With Contrast-Enhanced Mammography and Magnetic Resonance Imaging in the Assessment of Breast Lesions: A Pilot Study.Invest Radiol. 2025 Jun 1;60(6):369-375. doi: 10.1097/RLI.0000000000001138. Epub 2024 Dec 2. Invest Radiol. 2025. PMID: 39621875
-
Low-Dose, Contrast-Enhanced Mammography Compared to Contrast-Enhanced Breast MRI: A Feasibility Study.J Magn Reson Imaging. 2020 Aug;52(2):589-595. doi: 10.1002/jmri.27079. Epub 2020 Feb 14. J Magn Reson Imaging. 2020. PMID: 32061002 Free PMC article.
-
Preoperative evaluation of breast cancer: Contrast-enhanced mammography versus contrast-enhanced magnetic resonance imaging: A systematic review and meta-analysis.Breast Dis. 2022;41(1):303-315. doi: 10.3233/BD-210034. Breast Dis. 2022. PMID: 35754256
-
Contrast-enhanced mammography in high-dense breasts: a narrative review.Transl Breast Cancer Res. 2025 Mar 10;6:15. doi: 10.21037/tbcr-24-64. eCollection 2025. Transl Breast Cancer Res. 2025. PMID: 40421154 Free PMC article. Review.
Cited by
-
Advanced Magnetic Resonance Imaging Modalities for Breast Cancer Diagnosis: An Overview of Recent Findings and Perspectives.Diagnostics (Basel). 2022 Nov 9;12(11):2741. doi: 10.3390/diagnostics12112741. Diagnostics (Basel). 2022. PMID: 36359584 Free PMC article. Review.
-
Hormonal Regulation of Background Parenchymal Enhancement at Contrast-enhanced Mammography.Radiology. 2025 Feb;314(2):e241158. doi: 10.1148/radiol.241158. Radiology. 2025. PMID: 39932415
-
Radiomic Features Applied to Contrast Enhancement Spectral Mammography: Possibility to Predict Breast Cancer Molecular Subtypes in a Non-Invasive Manner.Int J Mol Sci. 2022 Dec 5;23(23):15322. doi: 10.3390/ijms232315322. Int J Mol Sci. 2022. PMID: 36499648 Free PMC article.
-
State-of-the-art for contrast-enhanced mammography.Br J Radiol. 2024 Mar 28;97(1156):695-704. doi: 10.1093/bjr/tqae017. Br J Radiol. 2024. PMID: 38374651 Free PMC article. Review.
-
Addition of contrast-enhanced mammography enhancement patterns and morphology for differentiating benign from malignant papillary breast lesions.Br J Radiol. 2025 Mar 1;98(1167):383-391. doi: 10.1093/bjr/tqae241. Br J Radiol. 2025. PMID: 39673439 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

