Impedimetric Detection Based on Label-Free Immunoassay Developed for Targeting Spike S1 Protein of SARS-CoV-2
- PMID: 36010342
- PMCID: PMC9407092
- DOI: 10.3390/diagnostics12081992
Impedimetric Detection Based on Label-Free Immunoassay Developed for Targeting Spike S1 Protein of SARS-CoV-2
Abstract
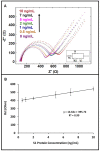
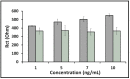
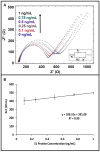
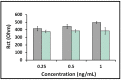
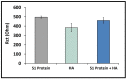
After the COVID-19 pandemic started all over the world, great importance was placed on the development of sensitive and selective bioanalytical assays for the rapid detection of the highly pathogenic SARS-CoV-2 virus causing COVID-19 disease. In this present work, an impedimetric immunosensor was developed and applied for rapid, reliable, sensitive and selective detection of the SARS-CoV-2 S1 protein. To detect the SARS-CoV-2 virus, targeting of the spike S1 protein was achieved herein by using S1 protein-specific capture antibody (Cab-S1) immobilized screen-printed electrode (SPE) in combination with the electrochemical impedance spectroscopy (EIS) technique. With the impedimetric immunosensor, the detection limit for S1 protein in buffer medium was found to be 0.23 ng/mL (equal to 23.92 amol in 8 µL sample) in the linear concentration range of S1 protein from 0.5 to 10 ng/mL. In the artificial saliva medium, it was found to be 0.09 ng/mL (equals to 9.36 amol in 8 µL sample) in the linear concentration range of S1 protein between 0.1 and 1 ng/mL. The selectivity of the impedimetric immunosensor toward S1 protein was tested against influenza hemagglutinin antigen (HA) in the buffer medium as well as in artificial saliva.
Keywords: COVID-19; SARS-CoV-2 S1 protein; electrochemical immunosensors; electrochemical impedance spectroscopy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. - DOI - PMC - PubMed
-
- Fabiani L., Saroglia M., Galatà G., De Santis R., Fillo S., Luca V., Faggioni G., D’Amore N., Regalbuto E., Salvatori P., et al. Magnetic beads combined with carbon black-based screen-printed electrodes for COVID-19: A reliable and miniaturized electrochemical immunosensor for SARS-CoV-2 detection in saliva. Biosens. Bioelectron. 2021;171:112686. doi: 10.1016/j.bios.2020.112686. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

