Artesunate Inhibits the Cell Growth in Colorectal Cancer by Promoting ROS-Dependent Cell Senescence and Autophagy
- PMID: 36010550
- PMCID: PMC9406496
- DOI: 10.3390/cells11162472
Artesunate Inhibits the Cell Growth in Colorectal Cancer by Promoting ROS-Dependent Cell Senescence and Autophagy
Abstract
Although artesunate has been reported to be a promising candidate for colorectal cancer (CRC) treatment, the underlying mechanisms and molecular targets of artesunate are yet to be explored. Here, we report that artesunate acts as a senescence and autophagy inducer to exert its inhibitory effect on CRC in a reactive oxygen species (ROS)-dependent manner. In SW480 and HCT116 cells, artesunate treatment led to mitochondrial dysfunction, drastically promoted mitochondrial ROS generation, and consequently inhibited cell proliferation by causing cell cycle arrest at G0/G1 phase as well as subsequent p16- and p21-mediated cell senescence. Senescent cells underwent endoplasmic reticulum stress (ERS), and the unfolded protein response (UPR) was activated via IRE1α signaling, with upregulated BIP, IRE1α, phosphorylated IRE1α (p-IRE1α), CHOP, and DR5. Further experiments revealed that autophagy was induced by artesunate treatment due to oxidative stress and ER stress. In contrast, N-Acetylcysteine (NAC, an ROS scavenger) and 3-Methyladenine (3-MA, an autophagy inhibitor) restored cell viability and attenuated autophagy in artesunate-treated cells. Furthermore, cellular free Ca2+ levels were increased and could be repressed by NAC, 3-MA, and GSK2350168 (an IRE1α inhibitor). In vivo, artesunate administration reduced the growth of CT26 cell-derived tumors in BALB/c mice. Ki67 and cyclin D1 expression was downregulated in tumor tissue, while p16, p21, p-IRE1α, and LC3B expression was upregulated. Taken together, artesunate induces senescence and autophagy to inhibit cell proliferation in colorectal cancer by promoting excessive ROS generation.
Keywords: artesunate; autophagy; cell senescence; colorectal cancer; reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



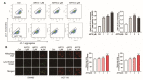
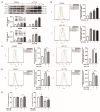


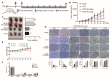
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

