RNA-Binding Proteins: Emerging Therapeutics for Vascular Dysfunction
- PMID: 36010571
- PMCID: PMC9407011
- DOI: 10.3390/cells11162494
RNA-Binding Proteins: Emerging Therapeutics for Vascular Dysfunction
Abstract
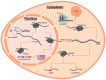
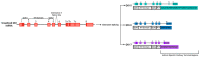
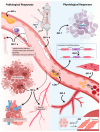
Vascular diseases account for a significant number of deaths worldwide, with cardiovascular diseases remaining the leading cause of mortality. This ongoing, ever-increasing burden has made the need for an effective treatment strategy a global priority. Recent advances in regenerative medicine, largely the derivation and use of induced pluripotent stem cell (iPSC) technologies as disease models, have provided powerful tools to study the different cell types that comprise the vascular system, allowing for a greater understanding of the molecular mechanisms behind vascular health. iPSC disease models consequently offer an exciting strategy to deepen our understanding of disease as well as develop new therapeutic avenues with clinical translation. Both transcriptional and post-transcriptional mechanisms are widely accepted to have fundamental roles in orchestrating responses to vascular damage. Recently, iPSC technologies have increased our understanding of RNA-binding proteins (RBPs) in controlling gene expression and cellular functions, providing an insight into the onset and progression of vascular dysfunction. Revelations of such roles within vascular disease states have therefore allowed for a greater clarification of disease mechanisms, aiding the development of novel therapeutic interventions. Here, we discuss newly discovered roles of RBPs within the cardio-vasculature aided by iPSC technologies, as well as examine their therapeutic potential, with a particular focus on the Quaking family of isoforms.
Keywords: QKI; Quaking; RNA-binding proteins; iPSCs; stem cell technologies; vascular disease.
Conflict of interest statement
Authors declare no conflict of interest.
Figures

References
-
- WHO Cardiovascular Diseases (CVDs) [(accessed on 30 June 2022)]. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases...
-
- Belair D., Whisler J.A., Valdez J., Velazquez J.J., Molenda J.A., Vickerman V., Lewis R., Daigh C., Hansen T.D., Mann D.A., et al. Human Vascular Tissue Models Formed from Human Induced Pluripotent Stem Cell Derived Endothelial Cells. Stem Cell Rev. Rep. 2015;11:511–525. doi: 10.1007/s12015-014-9549-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

