SARS-CoV-2 Spike Does Not Possess Intrinsic Superantigen-like Inflammatory Activity
- PMID: 36010602
- PMCID: PMC9406418
- DOI: 10.3390/cells11162526
SARS-CoV-2 Spike Does Not Possess Intrinsic Superantigen-like Inflammatory Activity
Abstract
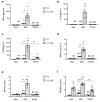
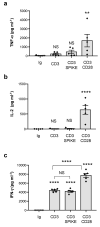
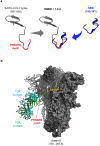
Multisystem inflammatory syndrome in children (MIS-C) is a rare hyperinflammatory disease occurring several weeks after SARS-CoV-2 infection. The clinical similarities between MIS-C and the toxic shock syndrome, together with the preferential expansion of T cells with a T-cell receptor variable β chain (TCRVβ) skewing, suggested a superantigen theory of MIS-C. For instance, recent in silico modelling evidenced the presence of a highly conserved motif within SARS-CoV-2 spike protein similar in structure to the superantigenic fragment of staphylococcal enterotoxin B (SEB). However, experimental data on the superantigenic activity of the SARS-CoV-2 spike have not yet been provided. Here, we assessed the superantigenic activity of the SARS-CoV-2 spike by analysing inflammatory cytokine production in both Jurkat cells and the peripheral blood CD4+ T cells stimulated with the SARS-CoV-2 spike or SEB as a control. We found that, unlike SEB, the SARS-CoV-2 spike does not exhibit an intrinsic superantigen-like activity.
Keywords: CD4+ T cells; MIS-C; SARS-CoV-2 spike; SEB; superantigen.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Multisystem Inflammatory Syndrome in Children and Long COVID: The SARS-CoV-2 Viral Superantigen Hypothesis.Front Immunol. 2022 Jul 7;13:941009. doi: 10.3389/fimmu.2022.941009. eCollection 2022. Front Immunol. 2022. PMID: 35874696 Free PMC article.
-
Superantigenic character of an insert unique to SARS-CoV-2 spike supported by skewed TCR repertoire in patients with hyperinflammation.Proc Natl Acad Sci U S A. 2020 Oct 13;117(41):25254-25262. doi: 10.1073/pnas.2010722117. Epub 2020 Sep 28. Proc Natl Acad Sci U S A. 2020. PMID: 32989130 Free PMC article.
-
A monoclonal antibody against staphylococcal enterotoxin B superantigen inhibits SARS-CoV-2 entry in vitro.Structure. 2021 Sep 2;29(9):951-962.e3. doi: 10.1016/j.str.2021.04.005. Epub 2021 Apr 29. Structure. 2021. PMID: 33930306 Free PMC article.
-
Immunology of Multisystem Inflammatory Syndrome after COVID-19 in Children: A Review of the Current Evidence.Int J Mol Sci. 2023 Mar 16;24(6):5711. doi: 10.3390/ijms24065711. Int J Mol Sci. 2023. PMID: 36982783 Free PMC article. Review.
-
Describing Elephants: An Update on the Immunopathology of Multisystem Inflammatory Syndrome in Children.Immunol Invest. 2024 Aug;53(6):962-974. doi: 10.1080/08820139.2024.2363833. Epub 2024 Jun 7. Immunol Invest. 2024. PMID: 38847319 Review.
Cited by
-
Bivalent binding of staphylococcal superantigens to the TCR and CD28 triggers inflammatory signals independently of antigen presenting cells.Front Immunol. 2023 May 3;14:1170821. doi: 10.3389/fimmu.2023.1170821. eCollection 2023. Front Immunol. 2023. PMID: 37207220 Free PMC article.
-
TGFβ links EBV to multisystem inflammatory syndrome in children.Nature. 2025 Apr;640(8059):762-771. doi: 10.1038/s41586-025-08697-6. Epub 2025 Mar 12. Nature. 2025. PMID: 40074901 Free PMC article.
-
More than a key-the pathological roles of SARS-CoV-2 spike protein in COVID-19 related cardiac injury.Sports Med Health Sci. 2023 Mar 30;6(3):209-20. doi: 10.1016/j.smhs.2023.03.004. Online ahead of print. Sports Med Health Sci. 2023. PMID: 37361919 Free PMC article. Review.
-
The identification of a SARs-CoV2 S2 protein derived peptide with super-antigen-like stimulatory properties on T-cells.Commun Biol. 2025 Jan 6;8(1):14. doi: 10.1038/s42003-024-07350-8. Commun Biol. 2025. PMID: 39762551 Free PMC article.
-
Molecular Mechanisms of Pathogenesis, Prevention, and Therapy of COVID-19: Summarizing the Results of 2021.Int J Mol Sci. 2022 Nov 17;23(22):14210. doi: 10.3390/ijms232214210. Int J Mol Sci. 2022. PMID: 36430684 Free PMC article.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

