The Mechanical Microenvironment Regulates Axon Diameters Visualized by Cryo-Electron Tomography
- PMID: 36010609
- PMCID: PMC9406316
- DOI: 10.3390/cells11162533
The Mechanical Microenvironment Regulates Axon Diameters Visualized by Cryo-Electron Tomography
Abstract
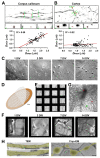
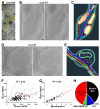
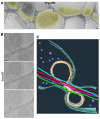
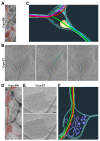
Axonal varicosities or swellings are enlarged structures along axon shafts and profoundly affect action potential propagation and synaptic transmission. These structures, which are defined by morphology, are highly heterogeneous and often investigated concerning their roles in neuropathology, but why they are present in the normal brain remains unknown. Combining confocal microscopy and cryo-electron tomography (Cryo-ET) with in vivo and in vitro systems, we report that non-uniform mechanical interactions with the microenvironment can lead to 10-fold diameter differences within an axon of the central nervous system (CNS). In the brains of adult Thy1-YFP transgenic mice, individual axons in the cortex displayed significantly higher diameter variation than those in the corpus callosum. When being cultured on lacey carbon film-coated electron microscopy (EM) grids, CNS axons formed varicosities exclusively in holes and without microtubule (MT) breakage, and they contained mitochondria, multivesicular bodies (MVBs), and/or vesicles, similar to the axonal varicosities induced by mild fluid puffing. Moreover, enlarged axon branch points often contain MT free ends leading to the minor branch. When the axons were fasciculated by mimicking in vivo axonal bundles, their varicosity levels reduced. Taken together, our results have revealed the extrinsic regulation of the three-dimensional ultrastructures of central axons by the mechanical microenvironment under physiological conditions.
Keywords: axon branch point; axon fasciculation; axonal varicosity; cryo-electron tomography (Cryo-ET); microtubule (MT); mitochondria; multivesicular body (MVB); primary neuron culture.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Partial interruption of axonal transport due to microtubule breakage accounts for the formation of periodic varicosities after traumatic axonal injury.Exp Neurol. 2012 Jan;233(1):364-72. doi: 10.1016/j.expneurol.2011.10.030. Epub 2011 Nov 4. Exp Neurol. 2012. PMID: 22079153 Free PMC article.
-
Immediate induction of varicosities by transverse compression but not uniaxial stretch in axon mechanosensation.Acta Neuropathol Commun. 2022 Jan 24;10(1):7. doi: 10.1186/s40478-022-01309-8. Acta Neuropathol Commun. 2022. PMID: 35074017 Free PMC article.
-
Morphology of mitochondria in spatially restricted axons revealed by cryo-electron tomography.PLoS Biol. 2018 Sep 17;16(9):e2006169. doi: 10.1371/journal.pbio.2006169. eCollection 2018 Sep. PLoS Biol. 2018. PMID: 30222729 Free PMC article.
-
Rapid and Reversible Development of Axonal Varicosities: A New Form of Neural Plasticity.Front Mol Neurosci. 2021 Feb 3;14:610857. doi: 10.3389/fnmol.2021.610857. eCollection 2021. Front Mol Neurosci. 2021. PMID: 33613192 Free PMC article. Review.
-
Cryo-electron tomography of cellular microtubules.Methods Cell Biol. 2010;97:455-73. doi: 10.1016/S0091-679X(10)97024-6. Methods Cell Biol. 2010. PMID: 20719285 Review.
Cited by
-
Membrane mechanics dictate axonal pearls-on-a-string morphology and function.Nat Neurosci. 2025 Jan;28(1):49-61. doi: 10.1038/s41593-024-01813-1. Epub 2024 Dec 2. Nat Neurosci. 2025. PMID: 39623218 Free PMC article.
-
Actomyosin-II protects axons from degeneration induced by mild mechanical stress.J Cell Biol. 2024 Aug 5;223(8):e202206046. doi: 10.1083/jcb.202206046. Epub 2024 May 7. J Cell Biol. 2024. PMID: 38713825 Free PMC article.
-
The Roles of Exosomes in Anti-Cancer Drugs.Cancer Med. 2025 May;14(9):e70897. doi: 10.1002/cam4.70897. Cancer Med. 2025. PMID: 40298189 Free PMC article. Review.
-
High-resolution spatiotemporal analysis of single serotonergic axons in an in vitro system.Front Neurosci. 2022 Oct 24;16:994735. doi: 10.3389/fnins.2022.994735. eCollection 2022. Front Neurosci. 2022. PMID: 36353595 Free PMC article.
-
An experimental platform for stochastic analyses of single serotonergic fibers in the mouse brain.Front Neurosci. 2023 Oct 6;17:1241919. doi: 10.3389/fnins.2023.1241919. eCollection 2023. Front Neurosci. 2023. PMID: 37869509 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

