Corneal Regeneration Using Adipose-Derived Mesenchymal Stem Cells
- PMID: 36010626
- PMCID: PMC9406486
- DOI: 10.3390/cells11162549
Corneal Regeneration Using Adipose-Derived Mesenchymal Stem Cells
Abstract
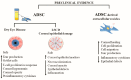
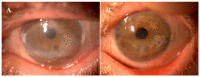
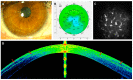
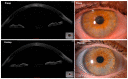
Adipose-derived stem cells are a subtype of mesenchymal stem cell that offers the important advantage of being easily obtained (in an autologous manner) from low invasive procedures, rendering a high number of multipotent stem cells with the potential to differentiate into several cellular lineages, to show immunomodulatory properties, and to promote tissue regeneration by a paracrine action through the secretion of extracellular vesicles containing trophic factors. This secretome is currently being investigated as a potential source for a cell-free based regenerative therapy for human tissues, which would significantly reduce the involved costs, risks and law regulations, allowing for a broader application in real clinical practice. In the current article, we will review the existing preclinical and human clinical evidence regarding the use of such adipose-derived mesenchymal stem cells for the regeneration of the three main layers of the human cornea: the epithelium (derived from the surface ectoderm), the stroma (derived from the neural crest mesenchyme), and the endothelium (derived from the neural crest cells).
Keywords: adipose-derived stem cells; cellular therapy; cornea; corneal epithelium; corneal regeneration; corneal stroma; corneal transplant; decellularized cornea; extracellular vesicles; mesenchymal stem cells; regenerative medicine; stem cells.
Conflict of interest statement
The authors, their families, their employers and their business associates have no financial or proprietary interest in any product or company associated with any device, instrument or drug mentioned in this article. The authors have not received any payment as consultants, reviewers or evaluators for any of the devices, instruments or drugs mentioned in this article.
Figures
References
-
- Blázquez-Martínez A., Chiesa M., Arnalich F., Fernandez F.A., Nistal M., De Miguel M. c-Kit identifies a subpopulation of mesenchymal stem cells in adipose tissue with higher telomerase expression and differentiation potential. Differentiation. 2014;87:147–160. doi: 10.1016/j.diff.2014.02.007. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

