Cellular Therapy Using Epitope-Imprinted Composite Nanoparticles to Remove α-Synuclein from an In Vitro Model
- PMID: 36010659
- PMCID: PMC9406856
- DOI: 10.3390/cells11162584
Cellular Therapy Using Epitope-Imprinted Composite Nanoparticles to Remove α-Synuclein from an In Vitro Model
Abstract
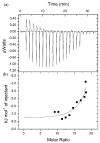
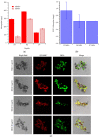
Several degenerative disorders of the central nervous system, including Parkinson's disease (PD), are related to the pathological aggregation of proteins. Antibodies against toxic disease proteins, such as α-synuclein (SNCA), are therefore being developed as possible therapeutics. In this work, one peptide (YVGSKTKEGVVHGVA) from SNCA was used as the epitope to construct magnetic molecularly imprinted composite nanoparticles (MMIPs). These composite nanoparticles were characterized by dynamic light scattering (DLS), high-performance liquid chromatography (HPLC), isothermal titration calorimetry (ITC), Brunauer-Emmett-Teller (BET) analysis, and superconducting quantum interference device (SQUID) analysis. Finally, the viability of brain endothelial cells that were treated with MMIPs was measured, and the extraction of SNCA from CRISPR/dCas9a-activated HEK293T cells from the in vitro model system was demonstrated for the therapeutic application of MMIPs.
Keywords: gene activation; magnetic nanoparticles; peptide imprinting; protein extraction; α-synuclein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Sorrentino Z.A., Goodwin M.S., Riffe C.J., Dhillon J.-K.S., Xia Y., Gorion K.-M., Vijayaraghavan N., McFarland K.N., Golbe L.I., Yachnis A.T., et al. Unique α-synuclein pathology within the amygdala in Lewy body dementia: Implications for disease initiation and progression. Acta Neuropathol. Commun. 2019;7:142. doi: 10.1186/s40478-019-0787-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

