Proteostasis Deregulation in Neurodegeneration and Its Link with Stress Granules: Focus on the Scaffold and Ribosomal Protein RACK1
- PMID: 36010666
- PMCID: PMC9406587
- DOI: 10.3390/cells11162590
Proteostasis Deregulation in Neurodegeneration and Its Link with Stress Granules: Focus on the Scaffold and Ribosomal Protein RACK1
Abstract
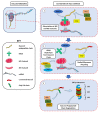
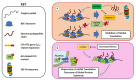
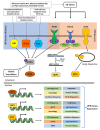
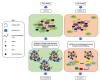
The role of protein misfolding, deposition, and clearance has been the dominant topic in the last decades of investigation in the field of neurodegeneration. The impairment of protein synthesis, along with RNA metabolism and RNA granules, however, are significantly emerging as novel potential targets for the comprehension of the molecular events leading to neuronal deficits. Indeed, defects in ribosome activity, ribosome stalling, and PQC-all ribosome-related processes required for proteostasis regulation-can contribute to triggering stress conditions and promoting the formation of stress granules (SGs) that could evolve in the formation of pathological granules, usually occurring during neurodegenerating effects. In this review, the interplay between proteostasis, mRNA metabolism, and SGs has been explored in a neurodegenerative context with a focus on Alzheimer's disease (AD), although some defects in these same mechanisms can also be found in frontotemporal dementia (FTD) and amyotrophic lateral sclerosis (ALS), which are discussed here. Finally, we highlight the role of the receptor for activated C kinase 1 (RACK1) in these pathologies and note that, besides its well characterized function as a scaffold protein, it has an important role in translation and can associate to stress granules (SGs) determining cell fate in response to diverse stress stimuli.
Keywords: RACK1; RNA; neurodegeneration; proteostasis; stress granules; translation.
Conflict of interest statement
The authors declare no conflict of interests.
Figures
Similar articles
-
Molecular interaction of stress granules with Tau and autophagy in Alzheimer's disease.Neurochem Int. 2022 Jul;157:105342. doi: 10.1016/j.neuint.2022.105342. Epub 2022 Apr 21. Neurochem Int. 2022. PMID: 35461975 Review.
-
Poly(GR) impairs protein translation and stress granule dynamics in C9orf72-associated frontotemporal dementia and amyotrophic lateral sclerosis.Nat Med. 2018 Aug;24(8):1136-1142. doi: 10.1038/s41591-018-0071-1. Epub 2018 Jun 25. Nat Med. 2018. PMID: 29942091 Free PMC article.
-
Stress granules in neurodegeneration--lessons learnt from TAR DNA binding protein of 43 kDa and fused in sarcoma.FEBS J. 2013 Sep;280(18):4348-70. doi: 10.1111/febs.12287. Epub 2013 May 9. FEBS J. 2013. PMID: 23587065 Review.
-
Lost in Translation: Evidence for Protein Synthesis Deficits in ALS/FTD and Related Neurodegenerative Diseases.Adv Neurobiol. 2018;20:283-301. doi: 10.1007/978-3-319-89689-2_11. Adv Neurobiol. 2018. PMID: 29916024 Free PMC article. Review.
-
RACK1 Regulates Poxvirus Protein Synthesis Independently of Its Role in Ribosome-Based Stress Signaling.J Virol. 2022 Sep 28;96(18):e0109322. doi: 10.1128/jvi.01093-22. Epub 2022 Sep 13. J Virol. 2022. PMID: 36098514 Free PMC article.
Cited by
-
The Labyrinthine Landscape of APP Processing: State of the Art and Possible Novel Soluble APP-Related Molecular Players in Traumatic Brain Injury and Neurodegeneration.Int J Mol Sci. 2023 Apr 2;24(7):6639. doi: 10.3390/ijms24076639. Int J Mol Sci. 2023. PMID: 37047617 Free PMC article.
-
Species-specific FMRP regulation of RACK1 is critical for prenatal cortical development.Neuron. 2023 Dec 20;111(24):3988-4005.e11. doi: 10.1016/j.neuron.2023.09.014. Epub 2023 Oct 10. Neuron. 2023. PMID: 37820724 Free PMC article.
-
Cancer Cells Evade Stress-Induced Apoptosis by Promoting HSP70-Dependent Clearance of Stress Granules.Cancers (Basel). 2022 Sep 25;14(19):4671. doi: 10.3390/cancers14194671. Cancers (Basel). 2022. PMID: 36230594 Free PMC article.
-
Decoding the molecular script of 2'-O-ribomethylation: Implications across CNS disorders.Heliyon. 2024 Oct 5;10(21):e39036. doi: 10.1016/j.heliyon.2024.e39036. eCollection 2024 Nov 15. Heliyon. 2024. PMID: 39524798 Free PMC article. Review.
-
Endocrine Disrupting Toxicity of Bisphenol A and Its Analogs: Implications in the Neuro-Immune Milieu.J Xenobiot. 2025 Jan 17;15(1):13. doi: 10.3390/jox15010013. J Xenobiot. 2025. PMID: 39846545 Free PMC article. Review.
References
-
- An W.-L., Cowburn R.F., Li L., Braak H., Alafuzoff I., Iqbal K., Iqbal I.-G., Winblad B., Pei J.-J. Up-Regulation of Phosphorylated/Activated p70 S6 Kinase and Its Relationship to Neurofibrillary Pathology in Alzheimer’s Disease. Am. J. Pathol. 2003;163:591–607. doi: 10.1016/S0002-9440(10)63687-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

