Developing a Reliable Holographic Flow Cyto-Tomography Apparatus by Optimizing the Experimental Layout and Computational Processing
- PMID: 36010667
- PMCID: PMC9406712
- DOI: 10.3390/cells11162591
Developing a Reliable Holographic Flow Cyto-Tomography Apparatus by Optimizing the Experimental Layout and Computational Processing
Abstract
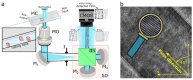
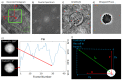
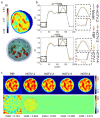
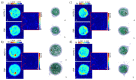
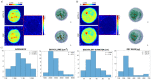
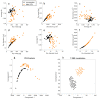
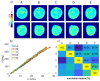
Digital Holographic Tomography (DHT) has recently been established as a means of retrieving the 3D refractive index mapping of single cells. To make DHT a viable system, it is necessary to develop a reliable and robust holographic apparatus in order that such technology can be utilized outside of specialized optics laboratories and operated in the in-flow modality. In this paper, we propose a quasi-common-path lateral-shearing holographic optical set-up to be used, for the first time, for DHT in a flow-cytometer modality. The proposed solution is able to withstand environmental vibrations that can severely affect the interference process. Furthermore, we have scaled down the system while ensuring that a full 360° rotation of the cells occurs in the field-of-view, in order to retrieve 3D phase-contrast tomograms of single cells flowing along a microfluidic channel. This was achieved by setting the camera sensor at 45° with respect to the microfluidic direction. Additional optimizations were made to the computational elements to ensure the reliable retrieval of 3D refractive index distributions by demonstrating an effective method of tomographic reconstruction, based on high-order total variation. The results were first demonstrated using realistic 3D numerical phantom cells to assess the performance of the proposed high-order total variation method in comparison with the gold-standard algorithm for tomographic reconstructions: namely, filtered back projection. Then, the proposed DHT system and the processing pipeline were experimentally validated for monocytes and mouse embryonic fibroblast NIH-3T3 cells lines. Moreover, the repeatability of these tomographic measurements was also investigated by recording the same cell multiple times and quantifying the ability to provide reliable and comparable tomographic reconstructions, as confirmed by a correlation coefficient greater than 95%. The reported results represent various steps forward in several key aspects of in-flow DHT, thus paving the way for its use in real-world applications.
Keywords: holographic microscopy; phase-contrast tomography; single-cells analysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

