Glycosylation in Renal Cell Carcinoma: Mechanisms and Clinical Implications
- PMID: 36010674
- PMCID: PMC9406705
- DOI: 10.3390/cells11162598
Glycosylation in Renal Cell Carcinoma: Mechanisms and Clinical Implications
Abstract
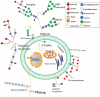
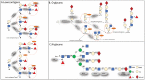
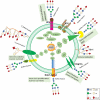
Renal cell carcinoma (RCC) is one of the most prevalent malignant tumors of the urinary system, accounting for around 2% of all cancer diagnoses and deaths worldwide. Clear cell RCC (ccRCC) is the most prevalent and aggressive histology with an unfavorable prognosis and inadequate treatment. Patients' progression-free survival is considerably improved by surgery; however, 30% of patients develop metastases following surgery. Identifying novel targets and molecular markers for RCC prognostic detection is crucial for more accurate clinical diagnosis and therapy. Glycosylation is a critical post-translational modification (PMT) for cancer cell growth, migration, and invasion, involving the transfer of glycosyl moieties to specific amino acid residues in proteins to form glycosidic bonds through the activity of glycosyltransferases. Most cancers, including RCC, undergo glycosylation changes such as branching, sialylation, and fucosylation. In this review, we discuss the latest findings on the significance of aberrant glycans in the initiation, development, and progression of RCC. The potential biomarkers of altered glycans for the diagnosis and their implications in RCC have been further highlighted.
Keywords: biomarkers; fucosylation; glycosylation; glycosyltransferase; renal cell carcinoma; sialylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

