Identification of Immune Cell Components in Breast Tissues by a Multiparametric Flow Cytometry Approach
- PMID: 36010863
- PMCID: PMC9406207
- DOI: 10.3390/cancers14163869
Identification of Immune Cell Components in Breast Tissues by a Multiparametric Flow Cytometry Approach
Abstract
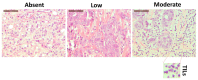
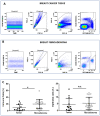
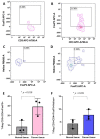
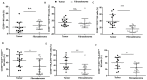
Immune cell components are able to infiltrate tumor tissues, and different reports described the presence of infiltrating immune cells (TILs) in several types of solid tumors, including breast cancer. The primary immune cell component cells are reported as a lymphocyte population mainly comprising the cytotoxic (CD8+) T cells, with varying proportions of helper (CD4+) T cells and CD19+ B cells, and rarely NK cells. In clinical practice, an expert pathologist commonly detects TILs areas in hematoxylin and eosin (H&E)-stained histological slides via light microscopy. Moreover, other more in-depth approaches could be used to better define the immunological component associated with tumor tissues. Using a multiparametric flow cytometry approach, we have studied the immune cells obtained from breast tumor tissues compared to benign breast pathologies. A detailed evaluation of immune cell components was performed on 15 and 14 biopsies obtained from breast cancer and fibroadenoma subjects, respectively. The percentage of tumor-infiltrating T lymphocytes was significantly higher in breast cancer patients compared to patients with fibroadenoma. Infiltrating helper T lymphocytes were increased in the case of malignant breast lesions, while cytotoxic T lymphocytes disclosed an opposite trend. In addition, our data suggest that the synergistic effect of the presence/activation of NK cells and NKT cells, in line with the data in the literature, determines the dampening of the immune response. Moreover, the lymphocyte-to-monocyte ratio was calculated and was completely altered in patients with breast cancer. Our approach could be a potent prognostic factor to be used in diagnostic/therapeutic purposes for the improvement of breast cancer patients' management.
Keywords: TILs; breast cancer; deep flow cytometry; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Pagès F., Kirilovsky A., Mlecnik B., Asslaber M., Tosolini M., Bindea G., Lagorce C., Wind P., Marliot F., Bruneval P., et al. In Situ Cytotoxic and Memory T Cells Predict Outcome in Patients With Early-Stage Colorectal Cancer. J. Clin. Oncol. 2009;27:5944–5951. doi: 10.1200/JCO.2008.19.6147. - DOI - PubMed
-
- Dieu-Nosjean M.-C., Antoine M., Danel C., Heudes D., Wislez M., Poulot V., Rabbe N., Laurans L., Tartour E., de Chaisemartin L., et al. Long-Term Survival for Patients With Non–Small-Cell Lung Cancer With Intratumoral Lymphoid Structures. J. Clin. Oncol. 2008;26:4410–4417. doi: 10.1200/JCO.2007.15.0284. - DOI - PubMed
-
- Denkert C., Loibl S., Noske A., Roller M., Müller B.M., Komor M., Budczies J., Darb-Esfahani S., Kronenwett R., Hanusch C., et al. Tumor-Associated Lymphocytes as an Independent Predictor of Response to Neoadjuvant Chemotherapy in Breast Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010;28:105–113. doi: 10.1200/JCO.2009.23.7370. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

