Grade 3-4 Immune-Related Adverse Events Induced by Immune Checkpoint Inhibitors in Non-Small-Cell Lung Cancer (NSCLC) Patients Are Correlated with Better Outcome: A Real-Life Observational Study
- PMID: 36010872
- PMCID: PMC9405595
- DOI: 10.3390/cancers14163878
Grade 3-4 Immune-Related Adverse Events Induced by Immune Checkpoint Inhibitors in Non-Small-Cell Lung Cancer (NSCLC) Patients Are Correlated with Better Outcome: A Real-Life Observational Study
Abstract
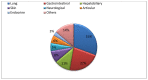
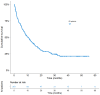
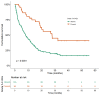
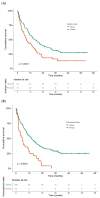
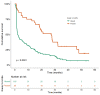
Background: Immune checkpoint inhibitors (ICIs) have been a major advance in treating non-small-cell lung cancer (NSCLC). Programmed cell death protein-1/programmed death-ligand 1 blockade enhances immune function, mediating anti-tumor activity, yet causing immune-related adverse events (irAEs). We investigated the prognostic role of Grade 3−4 irAEs on overall survival (OS). Methods: This observational study recruited advanced NSCLC patients who received ICIs at Bichat-Claude Bernard University Hospital and in a community hospital, Saint-Joseph Foundation (Paris), between 1 January 2016 and 31 December 2019. Immunotherapy as a single-agent or double-drug combination was applied in the first and later lines. Univariable and multivariable analyses were instrumental in evaluating the prognostic impact of irAEs. Results: Overall, 201 consecutive ICI-treated patients were enrolled. High-grade irAEs (Grades 3−4) occurred in 36 patients (17.9%), including 11 (30.5%) cases of pneumonitis, 8 (22.2%) of colitis, 4 (11.1%) hepatic, 3 (8.3%) dermatological, 2 (5.5%) neurological events, and 2 cases (5.5%) of poly-arthralgia. The median OS was 10.4 ± 1.36 months (95% CI:7.7−13.1), being significantly higher in patients with high-grade irAEs than those without, 27.8 months vs. 8.1 months, respectively (HR = 2.5; p < 0.0001). Multivariable analysis revealed an independent association between high-grade irAEs and longer OS (HR = 0.29, 95% CI: 0.2−0.6, p < 0.0001). Conclusions: Our real-life study confirms that high-grade irAEs predict longer OS in advanced NSCLC.
Keywords: immune checkpoint inhibitors; immune-related adverse events; immunotherapy; ipilimumab; nivolumab; non-small-cell lung cancer; pembrolizumab; prognosis.
Conflict of interest statement
V. Gounant reports personal fees from MSD, Chugai, Novartis, and Boehringer; personal fees and non-financial support from AstraZeneca, BMS, Takeda, and Pfizer; grants, personal fees, and non-financial support from Roche, all outside the submitted work. G. Zalcman reports personal fees from BMS, MSD, and Boehringer; non-financial support and other from Roche and AstraZeneca, as well as other support from Abbvie, all outside the submitted work. The other authors declared no conflict of interest with this study.
Figures

References
-
- Spini A., Gini R., Rosellini P., Singier A., Bellan C., Pascucci A., Leoncini L., Mathieu C., Martellucci I., Furiesi F., et al. First-Line Pharmacotherapies and Survival among Patients Diagnosed with Non-Resectable NSCLC: A Real-Life Setting Study with Gender Prospective. Cancers. 2021;13:6129. doi: 10.3390/cancers13236129. - DOI - PMC - PubMed
-
- Brahmer J., Reckamp K.L., Baas P., Crinò L., Eberhardt W.E.E., Poddubskaya E., Antonia S., Pluzanski A., Vokes E.E., Holgado E., et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

