Targeting MDM4 as a Novel Therapeutic Approach in Prostate Cancer Independent of p53 Status
- PMID: 36010941
- PMCID: PMC9405814
- DOI: 10.3390/cancers14163947
Targeting MDM4 as a Novel Therapeutic Approach in Prostate Cancer Independent of p53 Status
Abstract
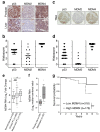
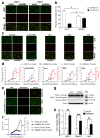
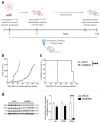
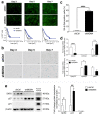
Metastatic prostate cancer is a lethal disease in patients incapable of responding to therapeutic interventions. Invasive prostate cancer spread is caused by failure of the normal anti-cancer defense systems that are controlled by the tumour suppressor protein, p53. Upon mutation, p53 malfunctions. Therapeutic strategies to directly re-empower the growth-restrictive capacities of p53 in cancers have largely been unsuccessful, frequently because of a failure to discriminate responses in diseased and healthy tissues. Our studies sought alternative prostate cancer drivers, intending to uncover new treatment targets. We discovered the oncogenic potency of MDM4 in prostate cancer cells, both in the presence and absence of p53 and also its mutation. We uncovered that sustained depletion of MDM4 is growth inhibitory in prostate cancer cells, involving either apoptosis or senescence, depending on the cell and genetic context. We identified that the potency of MDM4 targeting could be potentiated in prostate cancers with mutant p53 through the addition of a first-in-class small molecule drug that was selected as a p53 reactivator and has the capacity to elevate oxidative stress in cancer cells to drive their death.
Keywords: APR-246; MDM4; MDMX; TP53; XI-011; eprenetapopt; mutant p53; p53; prostate cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

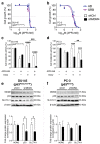
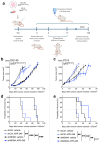
References
-
- Powell E., Piwnica-Worms D., Piwnica-Worms H. Contribution of p53 to metastasis. Cancer Discov. 2014;4:405–414. doi: 10.1158/2159-8290.CD-13-0136. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

