Combined Liquid Biopsy Methylation Analysis of CADM1 and MAL in Cervical Cancer Patients
- PMID: 36010947
- PMCID: PMC9406083
- DOI: 10.3390/cancers14163954
Combined Liquid Biopsy Methylation Analysis of CADM1 and MAL in Cervical Cancer Patients
Abstract
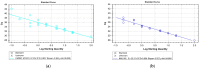
Cervical cancer is the fourth most common cancer in women, which is associated in >95% with a high-risk human papillomavirus (HPV) infection. Methylation of specific genes has been closely associated with the progress of cervical high-grade dysplastic lesions to invasive carcinomas. Therefore, DNA methylation has been proposed as a triage for women infected with high-risk HPV. Methylation analyses of cervical cancer tissue have shown that cell adhesion molecule 1 (CADM1) and myelin and lymphocyte protein (MAL) methylation are present in over 90% of all cervical high-grade neoplasias and invasive cervical cancers. Here, we established a liquid biopsy-based assay to detect MAL and CADM1 methylation in cell free (cf)DNA of cervical cancer. Methylation of the target gene was validated on bisulfite converted smear-DNA from cervical dysplasia patients and afterward applied to cfDNA using quantitative real-time PCR. In 52 smears, a combined analysis of CADM1 and/or MAL (CADM1/MAL) showed methylation in 86.5% of the cases. In cfDNA samples of 24 cervical cancer patients, CADM1/MAL methylation was detected in 83.3% of the cases. CADM1/MAL methylation was detected already in 81.8% of stage I-II patients showing the high sensitivity of this liquid biopsy assay. In combination with a specificity of 95.5% towards healthy donors (HD) and an area under the curve (AUC) of 0.872 in the receiver operating characteristic (ROC) analysis, CADM1/MAL cfDNA methylation detection might represent a novel and promising liquid biopsy marker in cervical cancer.
Keywords: CADM1; MAL; cervical cancer; liquid biopsy; methylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Ferlay J., Ervik M., Lam F., Colombet M., Mery L., Piñeros M., Znaor A., Soerjomataram I., Bray F. Global Cancer Observatory: Cancer Today. [(accessed on 6 July 2022)]. Available online: https://gco.iarc.fr/today.
-
- Arbyn M., Bryant A., Beutels P., Martin-Hirsch P.P., Paraskevaidis E., Van Hoof E., Steben M., Qiao Y., Zhao F.H., Schneider A., et al. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst. Rev. 2011;5:CD009069. doi: 10.1002/14651858.Cd009069. - DOI - PMC - PubMed
-
- Bruni L., Saura-Lázaro A., Montoliu A., Brotons M., Alemany L., Diallo M.S., Afsar O.Z., LaMontagne D.S., Mosina L., Contreras M., et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Prev. Med. 2021;144:106399. doi: 10.1016/j.ypmed.2020.106399. - DOI - PubMed
-
- World Health Organization . Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem. WHO; Geneva, Switzerland: 2020.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

