DWI Metrics Differentiating Benign Intraductal Papillary Mucinous Neoplasms from Invasive Pancreatic Cancer: A Study in GEM Models
- PMID: 36011011
- PMCID: PMC9406679
- DOI: 10.3390/cancers14164017
DWI Metrics Differentiating Benign Intraductal Papillary Mucinous Neoplasms from Invasive Pancreatic Cancer: A Study in GEM Models
Abstract
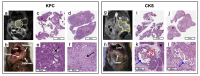
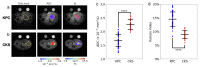
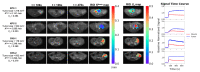
KPC (KrasG12D:Trp53R172H:Pdx1-Cre) and CKS (KrasG12D:Smad4L/L:Ptf1a-Cre) mice are genetically engineered mouse (GEM) models that capture features of human pancreatic ductal adenocarcinoma (PDAC) and intraductal papillary mucinous neoplasms (IPMN), respectively. We compared these autochthonous tumors using quantitative imaging metrics from diffusion-weighted MRI (DW-MRI) and dynamic contrast enhanced (DCE)-MRI in reference to quantitative histological metrics including cell density, fibrosis, and microvasculature density. Our results revealed distinct DW-MRI metrics between the KPC vs. CKS model (mimicking human PDAC vs. IPMN lesion): the apparent diffusion coefficient (ADC) of CKS tumors is significantly higher than that of KPC, with little overlap (mean ± SD 2.24±0.2 vs. 1.66±0.2, p<10−10) despite intratumor and intertumor variability. Kurtosis index (KI) is also distinctively separated in the two models. DW imaging metrics are consistent with growth pattern, cell density, and the cystic nature of the CKS tumors. Coregistration of ex vivo ADC maps with H&E-stained sections allowed for regional comparison and showed a correlation between local cell density and ADC value. In conclusion, studies in GEM models demonstrate the potential utility of diffusion-weighted MRI metrics for distinguishing pancreatic cancer from benign pancreatic cysts such as IPMN.
Keywords: diffusion-weighted MRI; dynamic contrast-enhanced MRI; genetically engineered mouse model; intraductal papillary mucinous neoplasms; neoplastic progression; pancreatic adenocarcinoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Hingorani S.R., Wang L., Multani A.S., Deramaudt R.H., Hruban R.H., Rustgi A.K., Chang S., Tuveson D.A. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. - DOI - PubMed
-
- Hingorani S.R., Petricoin E.F., Maitra A., Rajapakse V., King C., Jacobetz M.A., Ross S., Conrads T.P., Veenstra T.D., Hitt B.A., et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/S1535-6108(03)00309-X. - DOI - PubMed
-
- Bardeesy N., Cheng K.-H., Berger J.H., Chu G.C., Pahler J., Olson P., Hezel A.F., Horner J., Lauwers G.Y., Hanahan D., et al. Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes Dev. 2006;20:3130–3146. doi: 10.1101/gad.1478706. - DOI - PMC - PubMed
-
- Tanaka M., Fernandez-del Castillo C., Kamisawa T., Jang P., Levy P., Ohtsuka T., Saliva R., Shimizu Y., Tada M., Wolfgang C.L. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17:738–753. doi: 10.1016/j.pan.2017.07.007. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

