Tumour Derived Extracellular Vesicles: Challenging Target to Blunt Tumour Immune Evasion
- PMID: 36011012
- PMCID: PMC9406972
- DOI: 10.3390/cancers14164020
Tumour Derived Extracellular Vesicles: Challenging Target to Blunt Tumour Immune Evasion
Abstract
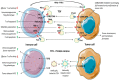
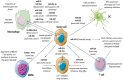
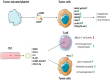
Control of the immune response is crucial for tumour onset and progression. Tumour cells handle the immune reaction by means of secreted factors and extracellular vesicles (EV). Tumour-derived extracellular vesicles (TEV) play key roles in immune reprogramming by delivering their cargo to different immune cells. Tumour-surrounding tissues also contribute to tumour immune editing and evasion, tumour progression, and drug resistance via locally released TEV. Moreover, the increase in circulating TEV has suggested their underpinning role in tumour dissemination. This review brings together data referring to TEV-driven immune regulation and antitumour immune suppression. Attention was also dedicated to TEV-mediated drug resistance.
Keywords: cell-to-cell communication; exosomes; extracellular vesicles; tumour antigens; tumour immune editing; tumour immune suppression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Myeloid Cell Modulation by Tumor-Derived Extracellular Vesicles.Int J Mol Sci. 2020 Aug 31;21(17):6319. doi: 10.3390/ijms21176319. Int J Mol Sci. 2020. PMID: 32878277 Free PMC article. Review.
-
Normoxic Tumour Extracellular Vesicles Modulate the Response of Hypoxic Cancer and Stromal Cells to Doxorubicin In Vitro.Int J Mol Sci. 2020 Aug 19;21(17):5951. doi: 10.3390/ijms21175951. Int J Mol Sci. 2020. PMID: 32824972 Free PMC article.
-
Extracellular vesicles as biomarkers in malignant pleural mesothelioma: A review.Crit Rev Oncol Hematol. 2020 Jun;150:102949. doi: 10.1016/j.critrevonc.2020.102949. Epub 2020 Apr 9. Crit Rev Oncol Hematol. 2020. PMID: 32330840 Review.
-
Extracellular Vesicles Work as a Functional Inflammatory Mediator Between Vascular Endothelial Cells and Immune Cells.Front Immunol. 2018 Aug 6;9:1789. doi: 10.3389/fimmu.2018.01789. eCollection 2018. Front Immunol. 2018. PMID: 30131806 Free PMC article.
-
IL-3 signalling in the tumour microenvironment shapes the immune response via tumour endothelial cell-derived extracellular vesicles.Pharmacol Res. 2022 May;179:106206. doi: 10.1016/j.phrs.2022.106206. Epub 2022 Apr 6. Pharmacol Res. 2022. PMID: 35398240
Cited by
-
Potential Pathophysiological Pathways in the Complex Relationships between OSA and Cancer.Cancers (Basel). 2023 Feb 7;15(4):1061. doi: 10.3390/cancers15041061. Cancers (Basel). 2023. PMID: 36831404 Free PMC article. Review.
-
Regulation of Extracellular Vesicle-Mediated Immune Responses against Antigen-Specific Presentation.Vaccines (Basel). 2022 Oct 10;10(10):1691. doi: 10.3390/vaccines10101691. Vaccines (Basel). 2022. PMID: 36298556 Free PMC article. Review.
-
Extracellular vesicles as tools and targets in therapy for diseases.Signal Transduct Target Ther. 2024 Feb 5;9(1):27. doi: 10.1038/s41392-024-01735-1. Signal Transduct Target Ther. 2024. PMID: 38311623 Free PMC article. Review.
-
The Experimental Study of Periodontal Ligament Stem Cells Derived Exosomes with Hydrogel Accelerating Bone Regeneration on Alveolar Bone Defect.Pharmaceutics. 2022 Oct 14;14(10):2189. doi: 10.3390/pharmaceutics14102189. Pharmaceutics. 2022. PMID: 36297624 Free PMC article.
References
-
- Slingluff C.L., Jr., Cox A.L., Stover J.M., Jr., Moore M.M., Hunt D.F., Engelhard V.H. Cytotoxic T-Lymphocyte Response to Autologous Human Squamous Cell Cancer of the Lung: Epitope Reconstitution with Peptides Extracted from HLA-Aw681. Cancer Res. 1994;54:2731–2737. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

