Adenosine Targeting as a New Strategy to Decrease Glioblastoma Aggressiveness
- PMID: 36011024
- PMCID: PMC9406358
- DOI: 10.3390/cancers14164032
Adenosine Targeting as a New Strategy to Decrease Glioblastoma Aggressiveness
Abstract
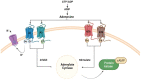
Glioblastoma is the most commonly malignant and aggressive brain tumor, with a high mortality rate. The role of the purine nucleotide adenosine and its interaction with its four subtypes receptors coupled to the different G proteins, A1, A2A, A2B, and A3, and its different physiological functions in different systems and organs, depending on the active receptor subtype, has been studied for years. Recently, several works have defined extracellular adenosine as a tumoral protector because of its accumulation in the tumor microenvironment. Its presence is due to both the interaction with the A2A receptor subtype and the increase in CD39 and CD73 gene expression induced by the hypoxic state. This fact has fueled preclinical and clinical research into the development of efficacious molecules acting on the adenosine pathway and blocking its accumulation. Given the success of anti-cancer immunotherapy, the new strategy is to develop selective A2A receptor antagonists that could competitively inhibit binding to its endogenous ligand, making them reliable candidates for the therapeutic management of brain tumors. Here, we focused on the efficacy of adenosine receptor antagonists and their enhancement in anti-cancer immunotherapy.
Keywords: A2AAR antagonist; adenosine; adenosine receptors; glioblastoma; immune evasion; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Bondy M.L., Scheurer M.E., Malmer B., Barnholtz-Sloan J.S., Davis F.G., Il’yasova D., Kruchko C., McCarthy B.J., Rajaraman P., Schwartzbaum J.A., et al. Brain tumor epidemiology: Consensus from the Brain Tumor Epidemiology Consortium. Cancer. 2008;113:1953–1968. doi: 10.1002/cncr.23741. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

