TNF Receptor Associated Factor 2 (TRAF2) Signaling in Cancer
- PMID: 36011046
- PMCID: PMC9406534
- DOI: 10.3390/cancers14164055
TNF Receptor Associated Factor 2 (TRAF2) Signaling in Cancer
Abstract
Tumor necrosis factor (TNF) receptor associated factor-2 (TRAF2) has been originally identified as a protein interacting with TNF receptor 2 (TNFR2) but also binds to several other receptors of the TNF receptor superfamily (TNFRSF). TRAF2, often in concert with other members of the TRAF protein family, is involved in the activation of the classical NFκB pathway and the stimulation of various mitogen-activated protein (MAP) kinase cascades by TNFRSF receptors (TNFRs), but is also required to inhibit the alternative NFκB pathway. TRAF2 has also been implicated in endoplasmic reticulum (ER) stress signaling, the regulation of autophagy, and the control of cell death programs. TRAF2 fulfills its functions by acting as a scaffold, bringing together the E3 ligase cellular inhibitor of apoptosis-1 (cIAP1) and cIAP2 with their substrates and various regulatory proteins, e.g., deubiquitinases. Furthermore, TRAF2 can act as an E3 ligase by help of its N-terminal really interesting new gene (RING) domain. The finding that TRAF2 (but also several other members of the TRAF family) interacts with the latent membrane protein 1 (LMP1) oncogene of the Epstein-Barr virus (EBV) indicated early on that TRAF2 could play a role in the oncogenesis of B-cell malignancies and EBV-associated non-keratinizing nasopharyngeal carcinoma (NPC). TRAF2 can also act as an oncogene in solid tumors, e.g., in colon cancer by promoting Wnt/β-catenin signaling. Moreover, tumor cell-expressed TRAF2 has been identified as a major factor-limiting cancer cell killing by cytotoxic T-cells after immune checkpoint blockade. However, TRAF2 can also be context-dependent as a tumor suppressor, presumably by virtue of its inhibitory effect on the alternative NFκB pathway. For example, inactivating mutations of TRAF2 have been associated with tumor development, e.g., in multiple myeloma and mantle cell lymphoma. In this review, we summarize the various TRAF2-related signaling pathways and their relevance for the oncogenic and tumor suppressive activities of TRAF2. Particularly, we discuss currently emerging concepts to target TRAF2 for therapeutic purposes.
Keywords: B-cell lymphoma; TNF receptor associated factor 2 (TRAF2); apoptosis; autophagy; cellular inhibitor of apoptosis 1/2 (cIAP1/2); necroptosis; nuclear factor ‘kappa-light-chain-enhancer’ of activated B-cells (NFκB); tumor necrosis factor (TNF).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
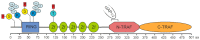



References
-
- Vince J.E., Pantaki D., Feltham R., Mace P.D., Cordier S.M., Schmukle A.C., Davidson A.J., Callus B.A., Wong W.W., Gentle I.E., et al. TRAF2 must bind to cellular inhibitors of apoptosis for tumor necrosis factor (tnf) to efficiently activate nf-{kappa}b and to prevent tnf-induced apoptosis. J. Biol. Chem. 2009;284:35906–35915. doi: 10.1074/jbc.M109.072256. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

