Appraisal for the Potential of Viral and Nonviral Vectors in Gene Therapy: A Review
- PMID: 36011281
- PMCID: PMC9407213
- DOI: 10.3390/genes13081370
Appraisal for the Potential of Viral and Nonviral Vectors in Gene Therapy: A Review
Abstract
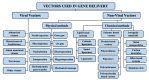
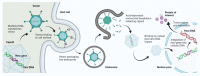
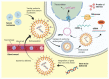
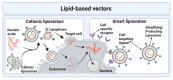
Over the past few decades, gene therapy has gained immense importance in medical research as a promising treatment strategy for diseases such as cancer, AIDS, Alzheimer's disease, and many genetic disorders. When a gene needs to be delivered to a target cell inside the human body, it has to pass a large number of barriers through the extracellular and intracellular environment. This is why the delivery of naked genes and nucleic acids is highly unfavorable, and gene delivery requires suitable vectors that can carry the gene cargo to the target site and protect it from biological degradation. To date, medical research has come up with two types of gene delivery vectors, which are viral and nonviral vectors. The ability of viruses to protect transgenes from biological degradation and their capability to efficiently cross cellular barriers have allowed gene therapy research to develop new approaches utilizing viruses and their different genomes as vectors for gene delivery. Although viral vectors are very efficient, science has also come up with numerous nonviral systems based on cationic lipids, cationic polymers, and inorganic particles that provide sustainable gene expression without triggering unwanted inflammatory and immune reactions, and that are considered nontoxic. In this review, we discuss in detail the latest data available on all viral and nonviral vectors used in gene delivery. The mechanisms of viral and nonviral vector-based gene delivery are presented, and the advantages and disadvantages of all types of vectors are also given.
Keywords: gene delivery; gene expression; gene therapy; nonviral vectors; transgene; viral vectors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

