microRNA Expression Profile of Purified Alveolar Epithelial Type II Cells
- PMID: 36011331
- PMCID: PMC9407429
- DOI: 10.3390/genes13081420
microRNA Expression Profile of Purified Alveolar Epithelial Type II Cells
Abstract
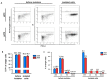
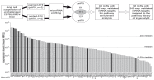
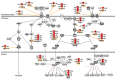
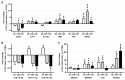
Alveolar type II (ATII) cells are essential for the maintenance of the alveolar homeostasis. However, knowledge of the expression of the miRNAs and miRNA-regulated networks which control homeostasis and coordinate diverse functions of murine ATII cells is limited. Therefore, we asked how miRNAs expressed in ATII cells might contribute to the regulation of signaling pathways. We purified "untouched by antibodies" ATII cells using a flow cytometric sorting method with a highly autofluorescent population of lung cells. TaqMan® miRNA low-density arrays were performed on sorted cells and intersected with miRNA profiles of ATII cells isolated according to a previously published protocol. Of 293 miRNAs expressed in both ATII preparations, 111 showed equal abundances. The target mRNAs of bona fide ATII miRNAs were used for pathway enrichment analysis. This analysis identified nine signaling pathways with known functions in fibrosis and/or epithelial-to-mesenchymal transition (EMT). In particular, a subset of 19 miRNAs was found to target 21 components of the TGF-β signaling pathway. Three of these miRNAs (miR-16-5p, -17-5p and -30c-5p) were down-modulated by TGF-β1 stimulation in human A549 cells, and concomitant up-regulation of associated mRNA targets (BMPR2, JUN, RUNX2) was observed. These results suggest an important role for miRNAs in maintaining the homeostasis of the TGF-β signaling pathway in ATII cells under physiological conditions.
Keywords: AECII; ATII; EMT; TGF-beta; alveolar epithelial type II cells; autofluorescence; flow cytometry; homeostasis; miRNAs; pathway analysis; type II pneumocytes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
The Regulation of Fatty Acid Synthase by Exosomal miR-143-5p and miR-342-5p in Idiopathic Pulmonary Fibrosis.Am J Respir Cell Mol Biol. 2024 Apr;70(4):259-282. doi: 10.1165/rcmb.2023-0232OC. Am J Respir Cell Mol Biol. 2024. PMID: 38117249 Free PMC article.
-
Histone deacetylase inhibitor restores surfactant protein-C expression in alveolar-epithelial type II cells and attenuates bleomycin-induced pulmonary fibrosis in vivo.Exp Lung Res. 2015;41(8):422-34. doi: 10.3109/01902148.2015.1060275. Epub 2015 Jul 7. Exp Lung Res. 2015. PMID: 26151196
-
MicroRNA-541-5p REgulates Type II Alveolar Epithelial Cell Proliferation and Activity by Modulating the HMGB1 Expression.Shock. 2022 Apr 1;57(4):536-543. doi: 10.1097/SHK.0000000000001852. Shock. 2022. PMID: 35271544 Free PMC article.
-
Mechanisms of ATII-to-ATI Cell Differentiation during Lung Regeneration.Int J Mol Sci. 2020 Apr 30;21(9):3188. doi: 10.3390/ijms21093188. Int J Mol Sci. 2020. PMID: 32366033 Free PMC article. Review.
-
The role of miRNAs in alveolar epithelial cells in emphysema.Biomed Pharmacother. 2021 Nov;143:112216. doi: 10.1016/j.biopha.2021.112216. Epub 2021 Sep 27. Biomed Pharmacother. 2021. PMID: 34649347 Free PMC article. Review.
Cited by
-
The Molecular Mechanisms and Function of miR-15a/16 Dysregulation in Fibrotic Diseases.Int J Mol Sci. 2022 Dec 16;23(24):16041. doi: 10.3390/ijms232416041. Int J Mol Sci. 2022. PMID: 36555676 Free PMC article. Review.
-
Ssc-miR-101-3p inhibits hypoxia-induced apoptosis and inflammatory response in alveolar type-II epithelial cells of Tibetan pigs via targeting FOXO3.Sci Rep. 2024 Aug 29;14(1):20124. doi: 10.1038/s41598-024-70510-7. Sci Rep. 2024. PMID: 39209907 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

