Oral Lesions Following Anti-SARS-CoV-2 Vaccination: A Systematic Review
- PMID: 36011863
- PMCID: PMC9408767
- DOI: 10.3390/ijerph191610228
Oral Lesions Following Anti-SARS-CoV-2 Vaccination: A Systematic Review
Abstract
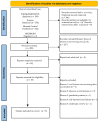
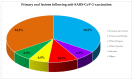
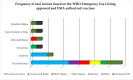
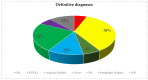
Increasing evidence relate anti-SARS-CoV-2 vaccinations to orofacial adverse reactions, therefore, the present systematic review aimed to evaluate primary oral lesions diagnosed in adult subjects, following the WHO Emergency Use Listing approved and EMA authorized vaccines, also in relation to cases' age, gender, comorbidities, and history of COVID-19, and in relation to vaccine type and doses. The study protocol, registered on PROSPERO (CRD42022339032) and compliant with the PRISMA statement, included an electronic search across Scopus, MEDLINE/PubMed, BioMed Central databases, and PROSPERO, ended on 18 June 2022 and succeeded by a manual search, an independent data extraction, and arisk of bias evaluation through ROBINS-I tool. Qualitatively synthesized data from the 13studies included showed an overall low prevalence (16 cases), though higher in females (68.8%), of oral lesions, mainly erosions and ulcers (34.5%). Nine cases were diagnosed following Pfizer-BioNTech, two Moderna, and one AstraZeneca, Serum Institute of India, Sinopharm, and Johnson&Johnson vaccines, respectively; specifically, eight after the first dose and seven after the second. In one case, vaccine type and dose were not specified. Considering newly developing vaccines, presented findings may be updated and further studies needed to highlight factors affecting oral lesion occurrence and specific macro-microscopic phenotypes in relation to cases' and vaccines' characteristics.
Keywords: COVID-19; SARS-CoV-2; coronavirus disease 2019; oral lesions; vaccination; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- European Database of Suspected Adverse Drug Reaction Reports-COVID 19 Message. [(accessed on 18 July 2022)]. Available online: http://www.adrreports.eu.
-
- Seirafianpour F., Pourriyahi H., Gholizadeh Mesgarha M., Pour Mohammad A., Shaka Z., Goodarzi A. A systematic review on mucocutaneous presentations after COVID-19 vaccination and expert recommendations about vaccination of important immune-mediated dermatologic disorders. Dermatol. Ther. 2022;35:e15461. doi: 10.1111/dth.15461. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

