Monitoring Anti-PEG Antibodies Level upon Repeated Lipid Nanoparticle-Based COVID-19 Vaccine Administration
- PMID: 36012103
- PMCID: PMC9408675
- DOI: 10.3390/ijms23168838
Monitoring Anti-PEG Antibodies Level upon Repeated Lipid Nanoparticle-Based COVID-19 Vaccine Administration
Abstract
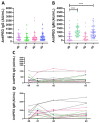
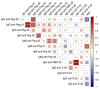
PEGylated lipids are one of the four constituents of lipid nanoparticle mRNA COVID-19 vaccines. Therefore, various concerns have been raised on the generation of anti-PEG antibodies and their potential role in inducing hypersensitivity reactions following vaccination or in reducing vaccine efficacy due to anti-carrier immunity. Here, we assess the prevalence of anti-PEG antibodies, in a cohort of vaccinated individuals, and give an overview of their time evolution after repeated vaccine administrations. Results indicate that, in our cohort, the presence of PEG in the formulation did not influence the level of anti-Spike antibodies generated upon vaccination and was not related to any reported, serious adverse effects. The time-course analysis of anti-PEG IgG showed no significant booster effect after each dose, whereas for IgM a significant increase in antibody levels was detected after the first and third dose. Data suggest that the presence of PEG in the formulation does not affect safety or efficacy of lipid-nanoparticle-based COVID-19 vaccines.
Keywords: COVID-19; SARS-CoV-2; anti-PEG Ig; anti-Spike Ig; lipid-nanoparticle-mRNA (LNP-mRNA)-based vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

