Terminal Phenoxy Group as a Privileged Moiety of the Drug Scaffold-A Short Review of Most Recent Studies 2013-2022
- PMID: 36012142
- PMCID: PMC9408176
- DOI: 10.3390/ijms23168874
Terminal Phenoxy Group as a Privileged Moiety of the Drug Scaffold-A Short Review of Most Recent Studies 2013-2022
Abstract
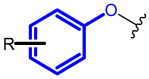
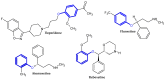
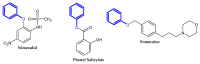
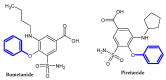
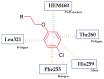
The terminal phenoxy group is a moiety of many drugs in use today. Numerous literature reports indicated its crucial importance for biological activity; thus, it is a privileged scaffold in medicinal chemistry. This review focuses on the latest achievements in the field of novel potential agents bearing a terminal phenoxy group in 2013-2022. The article provided information on neurological, anticancer, potential lymphoma agent, anti-HIV, antimicrobial, antiparasitic, analgesic, anti-diabetic as well as larvicidal, cholesterol esterase inhibitors, and antithrombotic or agonistic activities towards the adrenergic receptor. Additionally, for selected agents, the Structure-Activity-Relationship (SAR) is also discussed. Thus, this study may help the readers to better understand the nature of the phenoxy group, which will translate into rational drug design and the development of a more efficient drug. To the best of our knowledge, this is the first review devoted to an in-depth analysis of the various activities of compounds bearing terminal phenoxy moiety.
Keywords: adrenergic receptor activity; analgesic activity; anti-HIV activity; anticancer activity; antidiabetic; antimicrobial; drug scaffold; lymphoma; neurological disorder; phenoxy group.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




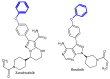

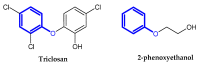

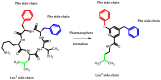

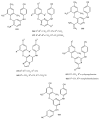
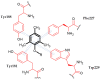

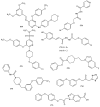
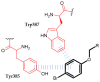

Similar articles
-
The Bioactivity of Thiazolidin-4-Ones: A Short Review of the Most Recent Studies.Int J Mol Sci. 2021 Oct 26;22(21):11533. doi: 10.3390/ijms222111533. Int J Mol Sci. 2021. PMID: 34768964 Free PMC article. Review.
-
Benzimidazole as a Privileged Scaffold in Drug Design and Discovery.Curr Top Med Chem. 2024;24(17):1504-1528. doi: 10.2174/0115680266314704240522112439. Curr Top Med Chem. 2024. PMID: 38818908 Review.
-
Thiazolopyrimidine, a privileged scaffold: Recent updates on synthetic and pharmacological perspective in drug discovery.Arch Pharm (Weinheim). 2025 Mar;358(3):e2400870. doi: 10.1002/ardp.202400870. Arch Pharm (Weinheim). 2025. PMID: 40123427 Review.
-
Recent advances in the chemistry and biology of benzothiazoles.Arch Pharm (Weinheim). 2015 Mar;348(3):155-78. doi: 10.1002/ardp.201400340. Epub 2015 Feb 12. Arch Pharm (Weinheim). 2015. PMID: 25682746 Review.
-
An Insight into the Synthesis and SAR of 2,4-Thiazolidinediones (2,4-TZD) as Multifunctional Scaffold: A Review.Mini Rev Med Chem. 2020;20(4):308-330. doi: 10.2174/1389557519666191029102838. Mini Rev Med Chem. 2020. PMID: 31660809 Review.
Cited by
-
Identification of Flavone Derivative Displaying a 4'-Aminophenoxy Moiety as Potential Selective Anticancer Agent in NSCLC Tumor Cells.Molecules. 2023 Apr 5;28(7):3239. doi: 10.3390/molecules28073239. Molecules. 2023. PMID: 37050002 Free PMC article.
-
Targeting cis-p-tau and neuro-related gene expression in traumatic brain injury: therapeutic insights from TC-DAPK6 treatment in mice.Mol Biol Rep. 2024 Sep 25;51(1):1010. doi: 10.1007/s11033-024-09945-0. Mol Biol Rep. 2024. PMID: 39320385
-
Anticancer Activity and Safety Profile of Novel 1-(4-Fluorophenoxyacetyl)-4-substituted Thio/Semicarbazide Derivatives.Molecules. 2025 Mar 31;30(7):1576. doi: 10.3390/molecules30071576. Molecules. 2025. PMID: 40286161 Free PMC article.
-
In Vitro and In Vivo Effects of Synthesis Novel Phenoxyacetamide Derivatives as Potent Apoptotic Inducer against HepG2 Cells through PARP-1 Inhibition.Pharmaceuticals (Basel). 2023 Oct 26;16(11):1524. doi: 10.3390/ph16111524. Pharmaceuticals (Basel). 2023. PMID: 38004390 Free PMC article.
-
Revisiting the Role of B-RAF Kinase as a Therapeutic Target in Melanoma.Curr Med Chem. 2024;31(15):2003-2020. doi: 10.2174/0109298673258495231011065225. Curr Med Chem. 2024. PMID: 37855341
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

