Alterations of Mitochondrial Structure in Methamphetamine Toxicity
- PMID: 36012188
- PMCID: PMC9408775
- DOI: 10.3390/ijms23168926
Alterations of Mitochondrial Structure in Methamphetamine Toxicity
Abstract
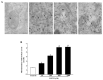
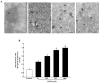
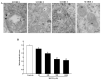
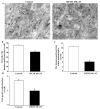
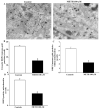
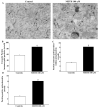
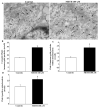
Recent evidence shows that methamphetamine (METH) produces mitochondrial alterations that contribute to neurotoxicity. Nonetheless, most of these studies focus on mitochondrial activity, whereas mitochondrial morphology remains poorly investigated. In fact, morphological evidence about the fine structure of mitochondria during METH toxicity is not available. Thus, in the present study we analyzed dose-dependent mitochondrial structural alterations during METH exposure. Light and transmission electron microscopy were used, along with ultrastructural stoichiometry of catecholamine cells following various doses of METH. In the first part of the study cell death and cell degeneration were assessed and they were correlated with mitochondrial alterations observed using light microscopy. In the second part of the study, ultrastructural evidence of specific mitochondrial alterations of crests, inner and outer membranes and matrix were quantified, along with in situ alterations of mitochondrial proteins. Neurodegeneration induced by METH correlates significantly with specific mitochondrial damage, which allows definition of a scoring system for mitochondrial integrity. In turn, mitochondrial alterations are concomitant with a decrease in fission/mitophagy protein Fis1 and DRP1 and an increase in Pink1 and Parkin in situ, at the mitochondrial level. These findings provide structural evidence that mitochondria represent both direct and indirect targets of METH-induced toxicity.
Keywords: DRP1; Fis1; MitoTracker; Parkin; Pink1; mitochondrial fission; mitophagy; neurotoxicity; psychostimulants; ultrastructural morphometry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
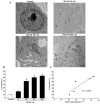
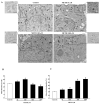
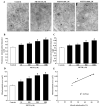
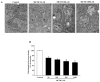
Similar articles
-
Loss of MIEF1/MiD51 confers susceptibility to BAX-mediated cell death and PINK1-PRKN-dependent mitophagy.Autophagy. 2019 Dec;15(12):2107-2125. doi: 10.1080/15548627.2019.1596494. Epub 2019 Mar 28. Autophagy. 2019. PMID: 30894073 Free PMC article.
-
A subcellular analysis of genetic modulation of PINK1 on mitochondrial alterations, autophagy and cell death.Arch Ital Biol. 2012 Jun-Sep;150(2-3):194-217. doi: 10.4449/aib.v150i2/3.1417. Arch Ital Biol. 2012. PMID: 23165879
-
Melatonin attenuates methamphetamine-induced disturbances in mitochondrial dynamics and degeneration in neuroblastoma SH-SY5Y cells.J Pineal Res. 2013 Oct;55(3):313-23. doi: 10.1111/jpi.12078. Epub 2013 Jul 27. J Pineal Res. 2013. PMID: 23889188
-
N-degron-mediated degradation and regulation of mitochondrial PINK1 kinase.Curr Genet. 2020 Aug;66(4):693-701. doi: 10.1007/s00294-020-01062-2. Epub 2020 Mar 10. Curr Genet. 2020. PMID: 32157382 Review.
-
Targeting mitochondrial dysfunction: role for PINK1 and Parkin in mitochondrial quality control.Antioxid Redox Signal. 2011 May 15;14(10):1929-38. doi: 10.1089/ars.2010.3799. Epub 2011 Mar 3. Antioxid Redox Signal. 2011. PMID: 21194381 Free PMC article. Review.
Cited by
-
Cleaving PINK1 or PGAM5? Involvement of PARL in Methamphetamine-Induced Excessive Mitophagy and Neuronal Necroptosis.CNS Neurosci Ther. 2025 Feb;31(2):e70293. doi: 10.1111/cns.70293. CNS Neurosci Ther. 2025. PMID: 40013375 Free PMC article.
-
Methamphetamine Increases Tubulo-Vesicular Areas While Dissipating Proteins from Vesicles Involved in Cell Clearance.Int J Mol Sci. 2024 Sep 4;25(17):9601. doi: 10.3390/ijms25179601. Int J Mol Sci. 2024. PMID: 39273545 Free PMC article.
-
Parents' Knowledge, Attitude, and Practices toward Methamphetamine Abuse among Youth and its Risk Factors in Saudi Arabia.J Pharm Bioallied Sci. 2024 Feb;16(Suppl 1):S753-S756. doi: 10.4103/jpbs.jpbs_996_23. Epub 2024 Jan 5. J Pharm Bioallied Sci. 2024. PMID: 38595546 Free PMC article.
-
Mechanisms and treatments of methamphetamine and HIV-1 co-induced neurotoxicity: a systematic review.Front Immunol. 2024 Aug 19;15:1423263. doi: 10.3389/fimmu.2024.1423263. eCollection 2024. Front Immunol. 2024. PMID: 39224601 Free PMC article.
-
Methamphetamine-Induced Blood Pressure Sensitization Correlates with Morphological Alterations within A1/C1 Catecholamine Neurons.Int J Mol Sci. 2024 Sep 24;25(19):10282. doi: 10.3390/ijms251910282. Int J Mol Sci. 2024. PMID: 39408612 Free PMC article.
References
-
- Moratalla R., Khairnar A., Simola N., Granado N., García-Montes J.R., Porceddu P.F., Tizabi Y., Costa G., Morelli M. Amphetamine-related drugs neurotoxicity in humans and in experimental animals: Main mechanisms. Prog. Neurobiol. 2017;155:149–170. doi: 10.1016/j.pneurobio.2015.09.011. - DOI - PubMed
-
- Limanaqi F., Busceti C.L., Celli R., Biagioni F., Fornai F. Autophagy as a gateway for the effects of methamphetamine: From neurotransmitter release and synaptic plasticity to psychiatric and neurodegenerative disorders. Prog. Neurobiol. 2021;204:102112. doi: 10.1016/j.pneurobio.2021.102112. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

