New Insights into Neutrophil Extracellular Trap (NETs) Formation from Porcine Neutrophils in Response to Bacterial Infections
- PMID: 36012224
- PMCID: PMC9409244
- DOI: 10.3390/ijms23168953
New Insights into Neutrophil Extracellular Trap (NETs) Formation from Porcine Neutrophils in Response to Bacterial Infections
Abstract
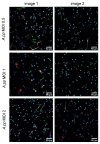
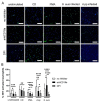
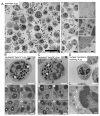
Actinobacillus pleuropneumoniae (A.pp, Gram negative) and Streptococcus (S.) suis (Gram positive) can cause severe diseases in pigs. During infection, neutrophils infiltrate to counteract these pathogens with phagocytosis and/or neutrophil extracellular traps (NETs). NETs consist of a DNA-backbone spiked with antimicrobial components. The NET formation mechanisms in porcine neutrophils as a response to both of the pathogens are not entirely clear. The aim of this study was to investigate whether A.pp (serotype 2, C3656/0271/11) and S. suis (serotype 2, strain 10) induce NETs by NADPH oxidase- or CD18-dependent mechanisms and to characterize phenotypes of NETs in porcine neutrophils. Therefore, we investigated NET induction in porcine neutrophils in the presence and absence of NET inhibitors and quantified NETs after 3 h. Furthermore, NETosis and phagocytosis were investigated by transmission electron microscopy after 30 min to characterize different phenotypes. A.pp and S. suis induce NETs that are mainly ROS-dependent. A.pp induces NETs that are partially CD18-dependent. Thirty minutes after infection, both of the pathogens induced a vesicular NET formation with only slight differences. Interestingly, some neutrophils showed only NET-marker positive phagolysosomes, but no NET-marker positive vesicles. Other neutrophils showed vesicular NETs and only NET-marker negative phagolysosomes. In conclusion, both of the pathogens induce ROS-dependent NETs. Vesicular NETosis and phagocytosis occur in parallel in porcine neutrophils in response to S. suis serotype 2 and A.pp serotype 2.
Keywords: Actinobacillus pleuropneumoniae (A.pp); Streptococcus (S.) suis; neutrophil extracellular traps (NETs); neutrophils; pigs; vesicular NETs.
Conflict of interest statement
Matthias Mörgelin is employed by Colzyx AB, Lund, Sweden. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Malech H.L., DeLeo F.R., Quinn M.T. The Role of Neutrophils in the Immune System: An Overview. Neutrophil. 2020;2087:3–10. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

