Functional Impairment of Endothelial Colony Forming Cells (ECFC) in Patients with Severe Atherosclerotic Cardiovascular Disease (ASCVD)
- PMID: 36012229
- PMCID: PMC9409296
- DOI: 10.3390/ijms23168969
Functional Impairment of Endothelial Colony Forming Cells (ECFC) in Patients with Severe Atherosclerotic Cardiovascular Disease (ASCVD)
Abstract
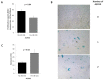
Endothelial dysfunction is a key factor in atherosclerosis. However, the link between endothelial repair and severity of atherosclerotic cardiovascular disease (ASCVD) is unclear. This study investigates the relationship between ASCVD, markers of inflammation, and circulating endothelial progenitor cells, namely hematopoietic cells with paracrine angiogenic activity and endothelial colony forming cells (ECFC). Two hundred and forty-three subjects from the TELARTA study were classified according to the presence of clinical atherosclerotic disease. ASCVD severity was assessed by the number of involved vascular territories. Flow cytometry was used to numerate circulating progenitor cells (PC) expressing CD34 and those co-expressing CD45, CD34, and KDR. Peripheral blood mononuclear cells ex vivo culture methods were used to determine ECFC and Colony Forming Unit- endothelial cells (CFU-EC). The ECFC subpopulation was analyzed for proliferation, senescence, and vasculogenic properties. Plasma levels of IL-6 and VEGF-A were measured using Cytokine Array. Despite an increased number of circulating precursors in ASCVD patients, ASCVD impaired the colony forming capacity and the angiogenic properties of ECFC in a severity-dependent manner. Alteration of ECFC was associated with increased senescent phenotype and IL-6 levels. Our study demonstrates a decrease in ECFC repair capacity according to ASCVD severity in an inflammatory and senescence-associated secretory phenotype context.
Keywords: atherosclerosis; endothelial progenitor cells; senescence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Hill J.M., Zalos G., Halcox J.P.J., Schenke W.H., Waclawiw M.A., Quyyumi A.A., Finkel T. Circulating Endothelial Progenitor Cells, Vascular Function, and Cardiovascular Risk. [(accessed on 15 December 2021)]. Available online: https://www.nejm.org/doi/10.1056/NEJMoa022287. - DOI - PubMed
-
- Werner N., Kosiol S., Schiegl T., Ahlers P., Walenta K., Link A., Böhm M., Nickenig G. Circulating Endothelial Progenitor Cells and Cardiovascular Outcomes. [(accessed on 15 December 2021)]. Available online: https://www.nejm.org/doi/10.1056/NEJMoa043814. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

