The Pivotal Role of NF-kB in the Pathogenesis and Therapeutics of Alzheimer's Disease
- PMID: 36012242
- PMCID: PMC9408758
- DOI: 10.3390/ijms23168972
The Pivotal Role of NF-kB in the Pathogenesis and Therapeutics of Alzheimer's Disease
Abstract
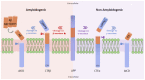
Alzheimer's Disease (AD) is the most common neurodegenerative disease worldwide, with a high prevalence that is expected to double every 20 years. Besides the formation of Aβ plaques and neurofibrillary tangles, neuroinflammation is one the major phenotypes that worsens AD progression. Indeed, the nuclear factor-κB (NF-κB) is a well-established inflammatory transcription factor that fuels neurodegeneration. Thus, in this review, we provide an overview of the NF-κB role in the pathogenesis of AD, including its interaction with various molecular factors in AD mice models, neurons, and glial cells. Some of these cell types and molecules include reactive microglia and astrocytes, β-secretase, APOE, glutamate, miRNA, and tau protein, among others. Due to the multifactorial nature of AD development and the failure of many drugs designed to dampen AD progression, the pursuit of novel targets for AD therapeutics, including the NF-κB signaling pathway, is rising. Herein, we provide a synopsis of the drug development landscape for AD treatment, offering the perspective that NF-κB inhibitors may generate widespread interest in AD research in the future. Ultimately, the additional investigation of compounds and small molecules that target NF-κB signaling and the complete understanding of NF-κB mechanistic activation in different cell types will broaden and provide more therapeutic options for AD patients.
Keywords: Alzheimer’s; NF-κB; drug discovery; inflammation; neurodegeneration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- NHS. [(accessed on 1 May 2022)]. Available online: https://www.nhs.uk/conditions/alzheimers-disease/
-
- Motolani A., Martin M., Sun M., Lu T. Reference Module in Biomed Science. Elsevier; Amsterdam, The Netherlands: 2021. NF-κB and Cancer Therapy Drugs.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

