The Relevance of Crystal Forms in the Pharmaceutical Field: Sword of Damocles or Innovation Tools?
- PMID: 36012275
- PMCID: PMC9408954
- DOI: 10.3390/ijms23169013
The Relevance of Crystal Forms in the Pharmaceutical Field: Sword of Damocles or Innovation Tools?
Abstract
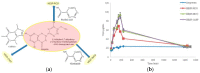
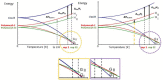
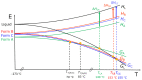
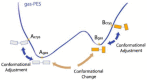
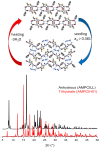
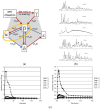
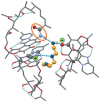
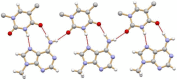
This review is aimed to provide to an "educated but non-expert" readership and an overview of the scientific, commercial, and ethical importance of investigating the crystalline forms (polymorphs, hydrates, and co-crystals) of active pharmaceutical ingredients (API). The existence of multiple crystal forms of an API is relevant not only for the selection of the best solid material to carry through the various stages of drug development, including the choice of dosage and of excipients suitable for drug development and marketing, but also in terms of intellectual property protection and/or extension. This is because the physico-chemical properties, such as solubility, dissolution rate, thermal stability, processability, etc., of the solid API may depend, sometimes dramatically, on the crystal form, with important implications on the drug's ultimate efficacy. This review will recount how the scientific community and the pharmaceutical industry learned from the catastrophic consequences of the appearance of new, more stable, and unsuspected crystal forms. The relevant aspects of hydrates, the most common pharmaceutical solid solvates, and of co-crystals, the association of two or more solid components in the same crystalline materials, will also be discussed. Examples will be provided of how to tackle multiple crystal forms with screening protocols and theoretical approaches, and ultimately how to turn into discovery and innovation the purposed preparation of new crystalline forms of an API.
Keywords: co-crystals of active pharmaceuticals; crystal polymorphism; hydrates.
Conflict of interest statement
The authors declare no conflict of interest.
Figures















References
-
- Mitscherlich E. Sur la relation qui existe entre la forme cristalline e le proportions chimiques, I. Memoires sur les arseniates et les phosphates. Ann. Chim. Phys. 1822;19:350–419.
-
- McCrone W.C. Physics and Chemistry of the Organic Solid State. In: Fox D., Labes M.M., Weissenberg A., editors. Physics Today. 2nd ed. Volume 2. Interscience; New York, NY, USA: 1965. 725p
-
- Bernstein J. Polymorphism in Molecular Crystals. 2nd ed. Oxford University Press; Oxford, UK: 2020.
-
- Brittain H.G., editor. Polymorphism in Pharmaceutical Solids. 2nd ed. Informa Healthcare; London, UK, USA: 1999.
-
- Hilfiker R., Von Raumer M., editors; Hilfiker R., Von Raumer M., editors. Polymorphism in the Pharmaceutical Industry: Solid Form and Drug Development. 2nd ed. Wiley-VCH; Weinheim, Germany: 2019.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

