Effect of Hydroxyapatite Coating by Er: YAG Pulsed Laser Deposition on the Bone Formation Efficacy by Polycaprolactone Porous Scaffold
- PMID: 36012313
- PMCID: PMC9409384
- DOI: 10.3390/ijms23169048
Effect of Hydroxyapatite Coating by Er: YAG Pulsed Laser Deposition on the Bone Formation Efficacy by Polycaprolactone Porous Scaffold
Abstract
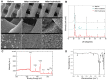
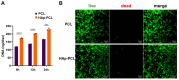
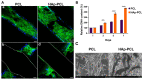
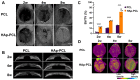
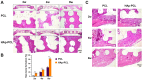
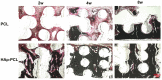
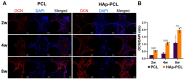
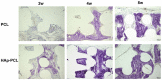
Composite scaffolds obtained by the combination of biodegradable porous scaffolds and hydroxyapatite with bone regeneration potential are feasible materials for bone tissue engineering. However, most composite scaffolds have been fabricated by complicated procedures or under thermally harsh conditions. We have previously demonstrated that hydroxyapatite coating onto various substrates under a thermally mild condition was achieved by erbium-doped yttrium aluminum garnet (Er: YAG) pulsed laser deposition (PLD). The purpose of this study was to prepare a polycaprolactone (PCL) porous scaffold coated with the hydroxyapatite by the Er: YAG-PLD method. Hydroxyapatite coating by the Er: YAG-PLD method was confirmed by morphology, crystallographic analysis, and surface chemical characterization studies. When cultured on PCL porous scaffold coated with hydroxyapatite, rat bone marrow-derived mesenchymal stem cells adhered, spread, and proliferated well. The micro-CT and staining analyses after the implantation of scaffold into the critical-sized calvaria bone defect in rats indicate that PCL porous scaffold coated with hydroxyapatite demonstrates accelerated and widespread bone formation. In conclusion, PCL porous scaffold coated with hydroxyapatite obtained by the Er: YAG-PLD method is a promising material in bone tissue engineering.
Keywords: Er: YAG laser; bone formation; hydroxyapatite coating; polycaprolactone; porous scaffold; pulsed laser deposition.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Effect of Er:YAG Pulsed Laser-Deposited Hydroxyapatite Film on Titanium Implants on M2 Macrophage Polarization In Vitro and Osteogenesis In Vivo.Int J Mol Sci. 2023 Dec 26;25(1):349. doi: 10.3390/ijms25010349. Int J Mol Sci. 2023. PMID: 38203519 Free PMC article.
-
A polycaprolactone/cuttlefish bone-derived hydroxyapatite composite porous scaffold for bone tissue engineering.J Biomed Mater Res B Appl Biomater. 2014 Jul;102(5):943-51. doi: 10.1002/jbm.b.33075. Epub 2013 Nov 21. J Biomed Mater Res B Appl Biomater. 2014. PMID: 24259295
-
Improvement of the compressive strength of a cuttlefish bone-derived porous hydroxyapatite scaffold via polycaprolactone coating.J Biomed Mater Res B Appl Biomater. 2013 Oct;101(7):1302-9. doi: 10.1002/jbm.b.32943. Epub 2013 May 10. J Biomed Mater Res B Appl Biomater. 2013. PMID: 23661509
-
Improvement of dual-leached polycaprolactone porous scaffolds by incorporating with hydroxyapatite for bone tissue regeneration.J Biomater Sci Polym Ed. 2014;25(17):1986-2008. doi: 10.1080/09205063.2014.966800. Epub 2014 Oct 7. J Biomater Sci Polym Ed. 2014. PMID: 25291106
-
Application of biodegradable Patient-specific scaffolds for maxillofacial bone regeneration: a scoping review of clinical studies.Br J Oral Maxillofac Surg. 2023 Nov;61(9):587-597. doi: 10.1016/j.bjoms.2023.08.215. Epub 2023 Sep 7. Br J Oral Maxillofac Surg. 2023. PMID: 37845099
Cited by
-
Impact of Hydroxyapatite on Gelatin/Oxidized Alginate 3D-Printed Cryogel Scaffolds.Gels. 2024 Jun 18;10(6):406. doi: 10.3390/gels10060406. Gels. 2024. PMID: 38920952 Free PMC article.
-
Effect of Er:YAG Pulsed Laser-Deposited Hydroxyapatite Film on Titanium Implants on M2 Macrophage Polarization In Vitro and Osteogenesis In Vivo.Int J Mol Sci. 2023 Dec 26;25(1):349. doi: 10.3390/ijms25010349. Int J Mol Sci. 2023. PMID: 38203519 Free PMC article.
-
Trends in bioactivity: inducing and detecting mineralization of regenerative polymeric scaffolds.J Mater Chem B. 2024 Mar 13;12(11):2720-2736. doi: 10.1039/d3tb02674d. J Mater Chem B. 2024. PMID: 38410921 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

