Structure-Dependent Toxicokinetics of Selected Pyrrolizidine Alkaloids In Vitro
- PMID: 36012484
- PMCID: PMC9408898
- DOI: 10.3390/ijms23169214
Structure-Dependent Toxicokinetics of Selected Pyrrolizidine Alkaloids In Vitro
Abstract
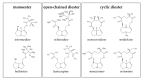
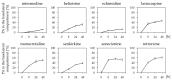
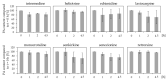
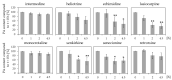
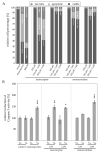
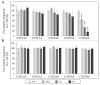
Phytochemicals like pyrrolizidine alkaloids (PAs) can affect the health of humans and animals. PAs can occur for example in tea, honey or herbs. Some PAs are known to be cytotoxic, genotoxic, and carcinogenic. Upon intake of high amounts, hepatotoxic and pneumotoxic effects were observed in humans. This study aims to elucidate different toxicokinetic parameters like the uptake of PAs and their metabolism with in vitro models. We examined the transport rates of differently structured PAs (monoester, open-chained diester, cyclic diester) over a model of the intestinal barrier. After passing the intestinal barrier, PAs reach the liver, where they are metabolized into partially instable electrophilic metabolites interacting with nucleophilic centers. We investigated this process by the usage of human liver, intestinal, and lung microsomal preparations for incubation with different PAs. These results are completed with the detection of apoptosis as indicator for bioactivation of the PAs. Our results show a structure-dependent passage of PAs over the intestinal barrier. PAs are structure-dependently metabolized by liver microsomes and, to a smaller extent, by lung microsomes. The detection of apoptosis of A549 cells treated with lasiocarpine and monocrotaline following bioactivation by human liver or lung microsomes underlines this result. Conclusively, our results help to shape the picture of PA toxicokinetics which could further improve the knowledge of molecular processes leading to observed effects of PAs in vivo.
Keywords: metabolism; pyrrolizidine alkaloids; structure-dependency; uptake.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Wiedenfeld H., Röder E., Bourauel T., Edgar J. Pyrrolizidine Alkaloids: Structure and Toxicity. V&R unipress GmbH; Goettingen, Germany: 2008.
-
- BfR (German Federal Institute for Risk Assessment) Aktualisierte Risikobewertung zu Gehalten an 1,2-ungesättigten Pyrrolizidinalkaloiden (PA) in Lebensmitteln. Stellungnahme 026/2020. BfR; Berlin, Germany: 2020. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

