Targeted Modification of Mammalian DNA by a Novel Type V Cas12a Endonuclease from Ruminococcus bromii
- PMID: 36012553
- PMCID: PMC9409102
- DOI: 10.3390/ijms23169289
Targeted Modification of Mammalian DNA by a Novel Type V Cas12a Endonuclease from Ruminococcus bromii
Abstract
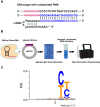
Type V Cas12a nucleases are DNA editors working in a wide temperature range and using expanded protospacer-adjacent motifs (PAMs). Though they are widely used, there is still a demand for discovering new ones. Here, we demonstrate a novel ortholog from Ruminococcus bromii sp. entitled RbCas12a, which is able to efficiently cleave target DNA templates, using the particularly high accessibility of PAM 5'-YYN and a relatively wide temperature range from 20 °C to 42 °C. In comparison to Acidaminococcus sp. (AsCas12a) nuclease, RbCas12a is capable of processing DNA more efficiently, and can be active upon being charged by spacer-only RNA at lower concentrations in vitro. We show that the human-optimized RbCas12a nuclease is also active in mammalian cells, and can be applied for efficient deletion incorporation into the human genome. Given the advantageous properties of RbCas12a, this enzyme shows potential for clinical and biotechnological applications within the field of genome editing.
Keywords: CRISPR; Cas endonuclease; genome editing; mammalian cells; site-directed mutagenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

