Involvement of Ferroptosis in Diabetes-Induced Liver Pathology
- PMID: 36012572
- PMCID: PMC9409200
- DOI: 10.3390/ijms23169309
Involvement of Ferroptosis in Diabetes-Induced Liver Pathology
Abstract
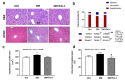
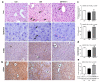
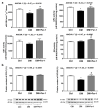
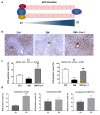
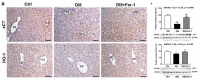
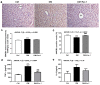
Cell death plays an important role in diabetes-induced liver dysfunction. Ferroptosis is a newly defined regulated cell death caused by iron-dependent lipid peroxidation. Our previous studies have shown that high glucose and streptozotocin (STZ) cause β-cell death through ferroptosis and that ferrostatin-1 (Fer-1), an inhibitor of ferroptosis, improves β-cell viability, islet morphology, and function. This study was aimed to examine in vivo the involvement of ferroptosis in diabetes-related pathological changes in the liver. For this purpose, male C57BL/6 mice, in which diabetes was induced with STZ (40 mg/kg/5 consecutive days), were treated with Fer-1 (1 mg/kg, from day 1-21 day). It was found that in diabetic mice Fer-1 improved serum levels of ALT and triglycerides and decreased liver fibrosis, hepatocytes size, and binucleation. This improvement was due to the Fer-1-induced attenuation of ferroptotic events in the liver of diabetic mice, such as accumulation of pro-oxidative parameters (iron, lipofuscin, 4-HNE), decrease in expression level/activity of antioxidative defense-related molecules (GPX4, Nrf2, xCT, GSH, GCL, HO-1, SOD), and HMGB1 translocation from nucleus into cytosol. We concluded that ferroptosis contributes to diabetes-related pathological changes in the liver and that the targeting of ferroptosis represents a promising approach in the management of diabetes-induced liver injury.
Keywords: Nrf2; diabetes; ferroptosis; lipid peroxidation; liver; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Marangiello R., Giorgetti R. A case of glycogenosis in a patient with insulin dependent diabetes. Minerva Pediatrica. 1996;48:279–281. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

