Differential Ganglioside and Cholesterol Depletion by Various Cyclodextrin Derivatives and Their Effect on Synaptosomal Glutamate Release
- PMID: 36012724
- PMCID: PMC9409351
- DOI: 10.3390/ijms23169460
Differential Ganglioside and Cholesterol Depletion by Various Cyclodextrin Derivatives and Their Effect on Synaptosomal Glutamate Release
Abstract
Gangliosides are glycosphingolipids of the plasma membrane and are highly enriched in the nervous system where they play a vital role in normal cell functions. Furthermore, several studies suggest their potential involvement in the pathogenesis of neurological conditions. Since cyclodextrins (CDs) can form inclusion complexes with various lipids, methylated beta-CDs are widely used in biomedical research to extract cholesterol from the membrane and study its cellular role. Despite CDs being known to interact with other membrane lipid components, their effect on gangliosides is poorly characterized. The aim of this research was to investigate the effect of dimethyl-beta-cyclodextrin (DIMEB), hydroxypropyl-beta-cyclodextrin (HPBCD), randomly methylated-alpha-cyclodextrin (RAMEA), and hydroxypropyl-alpha-cyclodextrin (HPACD) on ganglioside and cholesterol levels in rat brain synaptosomes. Their effect on membrane integrity and viability was also assessed. We examined the role of lipid depletion by CDs on the release of the major excitatory neurotransmitter, glutamate. Selective concentration range for cholesterol depletion was only found with HPBCD, but not with DIMEB. Selective depletion of gangliosides was achieved by both RAMEA and HPACD. The inhibition of stimulated glutamate release upon ganglioside depletion was found, suggesting their potential role in neurotransmission. Our study highlights the importance of the characterization of the lipid depleting capability of different CDs.
Keywords: cholesterol; cyclodextrines; gangliosides; glutamate release; lipid rafts; membrane lipids; membrane microdomains; synaptic transmission; synaptosomes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
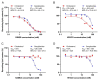
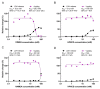
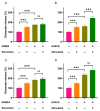
References
-
- Ma Q., Kobayashi M., Sugiura M., Ozaki N., Nishio K., Shiraishi Y., Furukawa K., Furukawa K., Sugiura Y. Morphological study of disordered myelination and the degeneration of nerve fibers in the spinal cord of mice lacking complex gangliosides. Arch. Histol. Cytol. 2003;66:37–44. doi: 10.1679/aohc.66.37. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

