Comparative Proteomics Study on the Postharvest Senescence of Volvariella volvacea
- PMID: 36012807
- PMCID: PMC9410126
- DOI: 10.3390/jof8080819
Comparative Proteomics Study on the Postharvest Senescence of Volvariella volvacea
Abstract
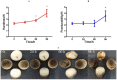
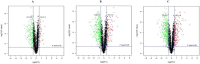
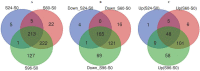
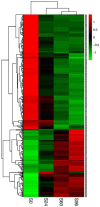
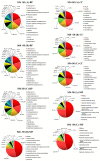
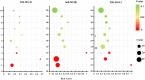
Volvariella volvacea is difficult to store after harvest, which restricts the production and circulation of V. volvacea fruiting bodies. Low-temperature storage is the traditional storage method used for most edible fungi. However, V. volvacea undergoes autolysis at low temperatures. When fruiting bodies are stored at 15 °C (suitable temperature), V. volvacea achieves the best fresh-keeping effect. However, the molecular mechanism underlying the postharvest senescence of V. volvacea remains unclear. Based on this information, we stored V. volvacea fruiting bodies at 15 °C after harvest and then analyzed the texture and phenotype combined with the results of previous physiological research. Four time points (0, 24, 60, and 96 h) were selected for the comparative proteomics study of V. volvacea during storage at 15 °C. A variety of proteins showed differential expressions in postharvest V. volvacea at 15 °C. Further comparison of the gene ontology (GO) enrichment analysis and KEGG pathways performed at different sampling points revealed proteins that were significantly enriched at several time points. At the same time, we also analyzed differentially expressed proteins (DEPs) related to the RNA transport, fatty acid biosynthesis and metabolism, and amino acid biosynthesis and metabolism pathways, and discussed the molecular functions of the PAB1, RPG1, ACC1, ADH3, ADH2, ALD5, and SDH2 proteins in postharvest V. volvacea senescence. Our results showed that many biological processes of the postharvest senescence of V. volvacea changed. Most importantly, we found that most RNA transport-related proteins were down-regulated, which may lead to a decrease in related gene regulation. Our results also showed that the expression of other important proteins, such as the fatty acid metabolism related proteins increased; and changes in fatty acid composition affected the cell membrane, which may accelerate the ripening and perception of V. volvacea fruiting bodies. Therefore, our research provides a reference for further studies on the aging mechanism of V. volvacea.
Keywords: V. volvacea; comparative proteomics; postharvest; senescence.
Conflict of interest statement
The authors have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper to declare.
Figures


References
-
- Paliyath G., Murr D.P., Handa A.K., Lurie S. Postharvest Biology and Technology of Fruits, Vegetables and Flowers. Wiley-Blackwell Publishing; New Delhi, India: 2008. pp. 154–196.
-
- Wu Z.L., Huang Y., Wang Z.J. Proteomic Analysis of V. volvacea in Response to Cold Stress. Food Sci. 2016;41:212–220. doi: 10.7506/spkx1002-6630-20190829-322. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

