Can Leukotriene Receptor Antagonist Therapy Improve the Control of Patients with Severe Asthma on Biological Therapy and Coexisting Bronchiectasis? A Pilot Study
- PMID: 36012941
- PMCID: PMC9410441
- DOI: 10.3390/jcm11164702
Can Leukotriene Receptor Antagonist Therapy Improve the Control of Patients with Severe Asthma on Biological Therapy and Coexisting Bronchiectasis? A Pilot Study
Abstract
Introduction: Asthma and bronchiectasis appear to be two related diseases and in their complex inflammatory interaction, the cysteinyl leukotriene/cysteinyl leukotriene receptor 1 (cysLT/cysLTR1) axis appears to play an important role given its involvement also in the neutrophilic pathway. To our knowledge, few studies have been conducted so far to evaluate the role of the leukotriene cysLT/cysLTr1 axis in the management of clinical and inflammatory outcomes within a population of patients with severe asthma and bronchiectasis. The aim of our study was to verify in this population the effect of leukotriene receptor antagonist (LTRA) therapy in clinical and inflammatory control before and after 6 months of introduction of biologic therapy.
Methods: We retrospectively enrolled, from eight different severe asthma centers' outpatients, 36 atopic patients with the simultaneous presence of non-cystic fibrosis (non-CF) and non-allergic bronchopulmonary aspergillosis (non-ABPA) bronchiectasis and severe asthma. The first biological injection was performed at baseline (T0 time). Patients who were already taking LTRA therapy at time T0 were recorded, and no new prescriptions were made. We observed our population over a 6-month period (T1 time). At the baseline we collected the following data: baseline characteristics, clinical history, high resolution computed tomography and bronchiectasis-related parameters and skin prick test. At both times T0 and T1 we collected the following data: asthma control test (ACT), asthma control questionnaire (ACQ), immunoglobulin E (IgE) level, blood count, fractional exhaled nitric oxide 50 (FeNO 50) and flow-volume spirometry. The study was retrospectively registered.
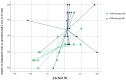
Results: Our population had a mean age of 59.08 ± 11.09 and 50% were female. At T1, patients on LTRA therapy had a significantly lower FeNO value (33.03 ± 23.61 vs. 88.92 ± 77.96; p = 0.012). We assessed that the value of ΔFeNO (FeNO 50 T1 - FeNO 50 T0) and the number of unplanned specialist visits allowed a discrimination of 66.7% in the presence of LTRA therapy. We also verified how low FeNO values at time T1 were statistically significant predictors of LTRA therapy (ODD = 9.96 (0.94-0.99); p = 0.032).
Conclusion: The presence of LTRA in therapy in a population of severe asthmatics with coexisting non-ASBPA bronchiectasis and non-cystic fibrosis, acting simultaneously on the T helper type 2 (TH2) pathway and probably on the neutrophilic component of bronchiectasis, would allow a further amplification of the beneficial effects of biological therapy, leading to a reduction in the number of unplanned visits to specialists.
Keywords: LTRA therapy; biological; bronchiectasis; severe asthma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Polverino E., Dimakou K., Hurst J., Martinez-Garcia M.A., Miravitlles M., Paggiaro P., Shteinberg M., Aliberti S., Chalmers J.D. The overlap between bronchiectasis and chronic airway diseases: State of the art and future directions. Eur. Respir. J. 2018;52:1800328. doi: 10.1183/13993003.00328-2018. - DOI - PubMed
-
- Bisaccioni C., Aun M.V., Cajuela E., Kalil J., Agondi R.C., Giavina-Bianchi P. Comorbidities in severe asthma: Frequency of rhinitis, nasal polyposis, gastroesophageal reflux disease, vocal cord dysfunction and bronchiectasis. Clinics. 2009;64:769–773. doi: 10.1590/S1807-59322009000800010. - DOI - PMC - PubMed
-
- Global Iniative for Asthma (GINA) 2022 Global Strategy for Asthma Management and Prevention, 2022. Update 2022. [(accessed on 13 July 2022)]. Available online: www.ginasthma.org.
-
- Polverino E., Goeminne P.C., McDonnell M.J., Aliberti S., Marshall S.E., Loebinger M.R., Murris M., Cantón R., Torres A., Dimakou K., et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur. Respir. J. 2017;50:1700629. doi: 10.1183/13993003.00629-2017. - DOI - PubMed

