Application of Machine Learning in Hospitalized Patients with Severe COVID-19 Treated with Tocilizumab
- PMID: 36012968
- PMCID: PMC9410189
- DOI: 10.3390/jcm11164729
Application of Machine Learning in Hospitalized Patients with Severe COVID-19 Treated with Tocilizumab
Abstract
Among the IL-6 inhibitors, tocilizumab is the most widely used therapeutic option in patients with SARS-CoV-2-associated severe respiratory failure (SRF). The aim of our study was to provide evidence on predictors of poor outcome in patients with COVID-19 treated with tocilizumab, using machine learning (ML) techniques. We conducted a retrospective study, analyzing the clinical, laboratory and sociodemographic data of patients admitted for severe COVID-19 with SRF, treated with tocilizumab. The extreme gradient boost (XGB) method had the highest balanced accuracy (93.16%). The factors associated with a worse outcome of tocilizumab use in terms of mortality were: baseline situation at the start of tocilizumab treatment requiring invasive mechanical ventilation (IMV), elevated ferritin, lactate dehydrogenase (LDH) and glutamate-pyruvate transaminase (GPT), lymphopenia, and low PaFi [ratio between arterial oxygen pressure and inspired oxygen fraction (PaO2/FiO2)] values. The factors associated with a worse outcome of tocilizumab use in terms of hospital stay were: baseline situation at the start of tocilizumab treatment requiring IMV or supplemental oxygen, elevated levels of ferritin, glutamate-oxaloacetate transaminase (GOT), GPT, C-reactive protein (CRP), LDH, lymphopenia, and low PaFi values. In our study focused on patients with severe COVID-19 treated with tocilizumab, the factors that were weighted most strongly in predicting worse clinical outcome were baseline status at the start of tocilizumab treatment requiring IMV and hyperferritinemia.
Keywords: COVID-19; SARS-CoV-2; cytokine release syndrome; machine learning; tocilizumab.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
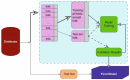
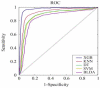
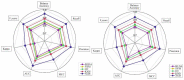
References
-
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. - DOI - PMC - PubMed
-
- Wang Z., Deng H., Ou C., Liang J., Wang Y., Jiang M., Li S. Clinical symptoms, comorbidities and complications in severe and non-severe patients with COVID-19: A systematic review and meta-analysis without cases duplication. Medicine. 2020;99:e23327. doi: 10.1097/MD.0000000000023327. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

