3D-Printing to Plan Complex Transcatheter Paravalvular Leaks Closure
- PMID: 36012997
- PMCID: PMC9410469
- DOI: 10.3390/jcm11164758
3D-Printing to Plan Complex Transcatheter Paravalvular Leaks Closure
Abstract
Background: Percutaneous closure of paravalvular leak (PVL) has emerged as an alternative to surgical management in selected cases. Achieving complete PVL occlusion, while respecting prosthesis function remains challenging. A multimodal imaging analysis of PVL morphology before and during the procedure is mandatory to select an appropriate device. We aim to explore the additional value of 3D printing in predicting device related adverse events including mechanical valve leaflet blockade, risk of device embolization and residual shunting.
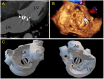
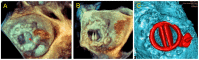
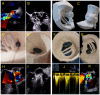
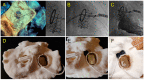
Methods: From the FFPP registries (NCT05089136 and NCT05117359), we included 11 transcatheter PVL closure procedures from three centers for which 3D printed models were produced. Cardiac CT was used for segmentation for 3D printed models (3D-heartmodeling, Caissargues, France). Technology used a laser to fuse very fine powders (TPU Thermoplastic polyurethane) into a final part-laser sintering technology (SLS) with an adapted elasticity. A simulation on 3D printed model was performed using a set of occluders.
Results: PVLs were located around aortic prostheses in six cases, mitral prostheses in four cases and tricuspid ring in one case. The device chosen during the simulation on the 3D printed model matched the one implanted in eight cases. In the three other cases, a similar device type was chosen during the procedures but with a different size. A risk of prosthesis leaflet blockade was identified on 3D printed models in four cases. During the procedure, the occluder was removed before release in one case. In another case the device was successfully repositioned and released. In two patients, leaflet impingement was observed post-operatively and surgical device removal had to be performed.
Conclusion: In a case-series of complex transcatheter PVL closure procedures, hands-on simulation testing on 3D printed models proved its usefulness to plan and facilitate these challenging procedures.
Keywords: 3D printing; interventional cardiology; multimodality imaging; paravalvular leak; percutaneous; prosthetic valve; transcatheter.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Otto C.M., Nishimura R.A., Bonow R.O., Carabello B.A., Erwin J.P., III, Gentile F., Jneid H., Krieger E.V., Mack M., McLeod C. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2021;77:450–500. doi: 10.1016/j.jacc.2020.11.035. - DOI - PubMed
-
- Vahanian A., Beyersdorf F., Praz F., Milojevic M., Baldus S., Bauersachs J., Capodanno D., Conradi L., De Bonis M., De Paulis R., et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Eur. Heart J. 2021;43:561–632. doi: 10.1093/eurheartj/ehab395. - DOI - PubMed
-
- Ruiz C.E., Hahn R.T., Berrebi A., Borer J.S., Cutlip D.E., Fontana G., Gerosa G., Ibrahim R., Jelnin V., Jilaihawi H., et al. Clinical Trial Principles and Endpoint Definitions for Paravalvular Leaks in Surgical Prosthesis. J. Am. Coll. Cardiol. 2017;69:2067–2087. doi: 10.1016/j.jacc.2017.02.038. - DOI - PubMed
-
- Calvert P.A., Northridge D.B., Malik I.S., Shapiro L., Ludman P., Qureshi S.A., Mullen M., Henderson R., Turner M., Been M., et al. Percutaneous Device Closure of Paravalvular Leak: Combined Experience From the United Kingdom and Ireland. Circulation. 2016;134:934–944. doi: 10.1161/CIRCULATIONAHA.116.022684. - DOI - PMC - PubMed
-
- García E., Arzamendi D., Jimenez-Quevedo P., Sarnago F., Martí G., Sanchez-Recalde A., Lasa-Larraya G., Sancho M., Iñiguez A., Goicolea J., et al. Outcomes and predictors of success and complications for paravalvular leak closure: An analysis of the SpanisH real-wOrld paravalvular LEaks closure (HOLE) registry. EuroIntervention. 2017;12:1962–1968. doi: 10.4244/EIJ-D-16-00581. - DOI - PubMed
LinkOut - more resources
Full Text Sources

