HPV-Positive and -Negative Cervical Cancers Are Immunologically Distinct
- PMID: 36013065
- PMCID: PMC9410291
- DOI: 10.3390/jcm11164825
HPV-Positive and -Negative Cervical Cancers Are Immunologically Distinct
Abstract
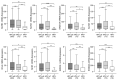
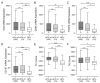
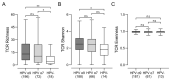
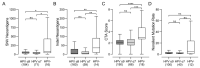
Although infection with human papillomavirus (HPV) is associated with nearly all cervical cancers (CC), a small proportion are HPV-negative. Recently, it has become clear that HPV-negative CC represent a distinct disease phenotype compared to HPV-positive disease and exhibit increased mortality. In addition, variations between different HPV types associated with CC have been linked to altered molecular pathology and prognosis. We compared the immune microenvironments of CC caused by HPV α9 species (HPV16-like), HPV α7 species (HPV18-like) and HPV-negative disease. HPV-negative CC appeared distinct from other subtypes, with greatly reduced levels of lymphocyte infiltration compared to either HPV α9 or α7 CC. Besides reduced levels of markers indicative of B, T, and NK lymphocytes, the expression of T-cell effector molecules, activation/exhaustion markers, and T-cell receptor diversity were also significantly lower in HPV-negative CC. Interestingly, HPV-negative CC expressed much higher levels of potential neoantigens than HPV-positive CC. These results identify profound differences between the immune landscape of HPV-positive and HPV-negative CC as well as modest differences between HPV α9 and α7 CC. These differences may contribute to altered patient outcomes between HPV-negative and HPV-positive CC and potentially between CC associated with different HPV types.
Keywords: T-cell function; TCGA; TCR repertoire; cervical cancer; gene expression; human papillomavirus; immune exhaustion; immune landscape; neoantigens; tumor immunology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- de Sanjose S., Quint W.G., Alemany L., Geraets D.T., Klaustermeier J.E., Lloveras B., Tous S., Felix A., Bravo L.E., Shin H.R., et al. Human papillomavirus genotype attribution in invasive cervical cancer: A retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–1056. doi: 10.1016/S1470-2045(10)70230-8. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

