Macrophages in Glioblastoma Development and Therapy: A Double-Edged Sword
- PMID: 36013403
- PMCID: PMC9409650
- DOI: 10.3390/life12081225
Macrophages in Glioblastoma Development and Therapy: A Double-Edged Sword
Abstract
Glioblastoma (GBM) is one of the leading lethal tumors, featuring aggressive malignancy and poor outcome to current standard temozolomide (TMZ) or radio-based therapy. Developing immunotherapies, especially immune checkpoint inhibitors, have improved patient outcomes in other solid tumors but remain fatigued in GBM patients. Emerging evidence has shown that GBM-associated macrophages (GAMs), comprising brain-resident microglia and bone marrow-derived macrophages, act critically in boosting tumor progression, altering drug resistance, and establishing an immunosuppressive environment. Based on its crucial role, evaluations of the safety and efficacy of GAM-targeted therapy are ongoing, with promising (pre)clinical evidence updated. In this review, we summarized updated literature related to GAM nature, the interplay between GAMs and GBM cells, and GAM-targeted therapeutic strategies.
Keywords: glioblastoma; immunotherapy; macrophage; microglia; targeted therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
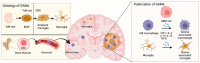
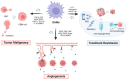
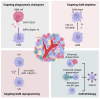
References
Publication types
Grants and funding
- no. 81873048/National Natural Science Foundation of China
- no. 2020JDJQ0065/Sichuan Provincial Science Fund for Distinguished Young Scholars of China
- no. ZYGX2020KYQD002/The Fundamental Research Funds for the Central Universities
- No. CELLPHYSIOL/SXMU-2021-CPOF202102/Open Fund from Key Laboratory of Cellular Physiology (Shanxi Medical University), Ministry of Education, China
- No. ZYGX2021YGCX004/Tunor Medical Technologies Innovation Fund of University of Electronic Science and Technology of China and Sichuan Cancer Center
LinkOut - more resources
Full Text Sources

