The Effect of Environment on the Evolution and Proliferation of Protocells of Increasing Complexity
- PMID: 36013406
- PMCID: PMC9410160
- DOI: 10.3390/life12081227
The Effect of Environment on the Evolution and Proliferation of Protocells of Increasing Complexity
Abstract
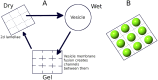
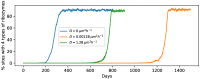
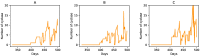
The formation, growth, division and proliferation of protocells containing RNA strands is an important step in ensuring the viability of a mixed RNA-lipid world. Experiments and computer simulations indicate that RNA encapsulated inside protocells can favor the protocell, promoting its growth while protecting the system from being over-run by selfish RNA sequences. Recent work has also shown that the rolling-circle replication mechanism can be harnessed to ensure the rapid growth of RNA strands and the probabilistic emergence and proliferation of protocells with functionally diverse ribozymes. Despite these advances in our understanding of a primordial RNA-lipid world, key questions remain about the ideal environment for the formation of protocells and its role in regulating the proliferation of functionally complex protocells. The hot spring hypothesis suggests that mineral-rich regions near hot springs, subject to dry-wet cycles, provide an ideal environment for the origin of primitive protocells. We develop a computational model to study protocellular evolution in such environments that are distinguished by the occurrence of three distinct phases, a wet phase, followed by a gel phase, and subsequently by a dry phase. We determine the conditions under which protocells containing multiple types of ribozymes can evolve and proliferate in such regions. We find that diffusion in the gel phase can inhibit the proliferation of complex protocells with the extent of inhibition being most significant when a small fraction of protocells is eliminated during environmental cycling. Our work clarifies how the environment can shape the evolution and proliferation of complex protocells.
Keywords: RNA world; evolution; hot spring hypothesis; origin of life; primordial environment; protocell; ribozymes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources

