A Contextual-Bandit-Based Approach for Informed Decision-Making in Clinical Trials
- PMID: 36013456
- PMCID: PMC9410371
- DOI: 10.3390/life12081277
A Contextual-Bandit-Based Approach for Informed Decision-Making in Clinical Trials
Abstract
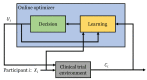
Clinical trials are conducted to evaluate the efficacy of new treatments. Clinical trials involving multiple treatments utilize the randomization of treatment assignments to enable the evaluation of treatment efficacies in an unbiased manner. Such evaluation is performed in post hoc studies that usually use supervised-learning methods that rely on large amounts of data collected in a randomized fashion. That approach often proves to be suboptimal in that some participants may suffer and even die as a result of having not received the most appropriate treatments during the trial. Reinforcement-learning methods improve the situation by making it possible to learn the treatment efficacies dynamically during the course of the trial, and to adapt treatment assignments accordingly. Recent efforts using multi-arm bandits, a type of reinforcement-learning method, have focused on maximizing clinical outcomes for a population that was assumed to be homogeneous. However, those approaches have failed to account for the variability among participants that is becoming increasingly evident as a result of recent clinical-trial-based studies. We present a contextual-bandit-based online treatment optimization algorithm that, in choosing treatments for new participants in the study, takes into account not only the maximization of the clinical outcomes as well as the patient characteristics. We evaluated our algorithm using a real clinical trial dataset from the International Stroke Trial. We simulated the online setting by sequentially going through the data of each participant admitted to the trial. Two bandits (one for each context) were created, with four choices of treatments. For a new participant in the trial, depending on the context, one of the bandits was selected. Then, we took three different approaches to choose a treatment: (a) a random choice (i.e., the strategy currently used in clinical trial settings), (b) a Thompson sampling-based approach, and (c) a UCB-based approach. Success probabilities of each context were calculated separately by considering the participants with the same context. Those estimated outcomes were used to update the prior distributions within the bandit corresponding to the context of each participant. We repeated that process through the end of the trial and recorded the outcomes and the chosen treatments for each approach. We also evaluated a context-free multi-arm-bandit-based approach, using the same dataset, to showcase the benefits of our approach. In the context-free case, we calculated the success probabilities for the Bernoulli sampler using the whole clinical trial dataset in a context-independent manner. The results of our retrospective analysis indicate that the proposed approach performs significantly better than either a random assignment of treatments (the current gold standard) or a multi-arm-bandit-based approach, providing substantial gains in the percentage of participants who are assigned the most suitable treatments. The contextual-bandit and multi-arm bandit approaches provide 72.63% and 64.34% gains, respectively, compared to a random assignment.
Keywords: bandits; clinical trials; machine learning.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
An empirical evaluation of active inference in multi-armed bandits.Neural Netw. 2021 Dec;144:229-246. doi: 10.1016/j.neunet.2021.08.018. Epub 2021 Aug 26. Neural Netw. 2021. PMID: 34507043
-
A Multiplier Bootstrap Approach to Designing Robust Algorithms for Contextual Bandits.IEEE Trans Neural Netw Learn Syst. 2023 Dec;34(12):9887-9899. doi: 10.1109/TNNLS.2022.3161806. Epub 2023 Nov 30. IEEE Trans Neural Netw Learn Syst. 2023. PMID: 35385392
-
Multi-Armed Bandits in Brain-Computer Interfaces.Front Hum Neurosci. 2022 Jul 5;16:931085. doi: 10.3389/fnhum.2022.931085. eCollection 2022. Front Hum Neurosci. 2022. PMID: 35874164 Free PMC article. Review.
-
Nonsomatic treatment of depression.Child Adolesc Psychiatr Clin N Am. 2002 Jul;11(3):579-93. doi: 10.1016/s1056-4993(02)00009-3. Child Adolesc Psychiatr Clin N Am. 2002. PMID: 12222084 Review.
Cited by
-
Machine learning for clinical trials in the era of COVID-19.Stat Biopharm Res. 2020 Aug 18;12(4):506-517. doi: 10.1080/19466315.2020.1797867. Stat Biopharm Res. 2020. PMID: 34191983 Free PMC article.
-
Machine learning applications in drug development.Comput Struct Biotechnol J. 2019 Dec 26;18:241-252. doi: 10.1016/j.csbj.2019.12.006. eCollection 2020. Comput Struct Biotechnol J. 2019. PMID: 33489002 Free PMC article. Review.
References
-
- Fuster V., Bhatt D.L., Califf R.M., Michelson A.D., Sabatine M.S., Angiolillo D.J., Bates E.R., Cohen D.J., Coller B.S., Furie B., et al. Guided antithrombotic therapy: Current status and future research direction: Report on a National Heart, Lung and Blood Institute working group. Circulation. 2012;126:1645–1662. doi: 10.1161/CIRCULATIONAHA.112.105908. - DOI - PMC - PubMed
-
- Coffey C.S., Kairalla J.A. Adaptive clinical trials. Drugs R & D. 2008;9:229–242. - PubMed
LinkOut - more resources
Full Text Sources

