Changes of Estimated Glomerular Filtration Rate and Glycated Hemoglobin A1c in Diabetic Macular Edema Patients Treated by Ranibizumab and Aflibercept in the Tertiary Referral Hospital
- PMID: 36013548
- PMCID: PMC9414450
- DOI: 10.3390/medicina58081081
Changes of Estimated Glomerular Filtration Rate and Glycated Hemoglobin A1c in Diabetic Macular Edema Patients Treated by Ranibizumab and Aflibercept in the Tertiary Referral Hospital
Abstract
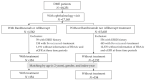
Background and Objectives: Intravitreal injections (IVI) of vascular endothelial growth factor (VEGF) inhibitors are guideline-indicated treatments for diabetic macular edema (DME). However, some recent data have suggested that IVI VEGF inhibitors might, through systemic absorption, lead to a reduction in renal function. Our study aims to compare changes in glycated hemoglobin A1c (HbA1c) and estimated glomerular filtration rate (eGFR) between patients who received IVI ranibizumab and aflibercept treatment and patients who have not received IVI treatments. Materials and Methods: There were 17,165 DME patients with documented ophthalmology visits in the China Medical University Hospital-Clinical Research Data Repository. Those with a history of ESRD or bevacizumab treatment history, and those with missing information on HbA1c or eGFR, were excluded. After matching by age (±2 years), gender, and the year of clinical visit, 154 patients with medical treatment (including ranibizumab and aflibercept) and 154 patients without medical treatment were included in the study. The difference between HbA1c and eGFR at baseline and 3 and 12 months after the index date between the two groups was assessed. Results: Mean HbA1c and eGFR decreased between baseline and 12 months after the index date in both groups (p < 0.05). Compared with the non-treatment group, the treatment group had significantly lower HbA1c 3 and 12 months after the index date. There was no significant difference in eGFR between the two groups. In the generalized estimating equations (GEE) model, HbA1c in the treatment group was lower than the non-treatment group (−0.44%, 95% CI = −0.75, −0.14), but eGFR was similar after adjusting for age, gender, and index-year. HbA1c and eGFR decreased with the time in the adjusted GEE model (p < 0.0001) in both groups. Conclusions: This study showed that eGFR decreased with age and time and was not related to IVI anti-VEGF treatments in our tertiary referral hospital. IVI anti-VEGF therapy was also associated with better HbA1c control. It is suggested that DME patients can receive intravitreal VEGF inhibitors without inducing more renal impairment.
Keywords: DME; HbA1c; IVI; aflibercept; anti-VEGF; eGFR; intravitreal injections; ranibizumab.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
CHANGES IN PLASMA VASCULAR ENDOTHELIAL GROWTH FACTOR LEVEL AFTER INTRAVITREAL INJECTION OF BEVACIZUMAB, AFLIBERCEPT, OR RANIBIZUMAB FOR DIABETIC MACULAR EDEMA.Retina. 2018 Sep;38(9):1801-1808. doi: 10.1097/IAE.0000000000002004. Retina. 2018. PMID: 29280940 Free PMC article. Clinical Trial.
-
Therapeutic effect of cataract surgery with simultaneous intravitreal injection of aflibercept on diabetic macular edema: An observational study.Medicine (Baltimore). 2022 Aug 19;101(33):e30115. doi: 10.1097/MD.0000000000030115. Medicine (Baltimore). 2022. PMID: 35984152 Free PMC article.
-
Comparison of Aflibercept, Bevacizumab, and Ranibizumab for Treatment of Diabetic Macular Edema: Extrapolation of Data to Clinical Practice.JAMA Ophthalmol. 2016 Jan;134(1):95-9. doi: 10.1001/jamaophthalmol.2015.4110. JAMA Ophthalmol. 2016. PMID: 26512939
-
Treatment strategies for refractory diabetic macular edema: switching anti-VEGF treatments, adopting corticosteroid-based treatments, and combination therapy.Expert Opin Biol Ther. 2016;16(3):365-74. doi: 10.1517/14712598.2016.1131265. Epub 2016 Jan 12. Expert Opin Biol Ther. 2016. PMID: 26674182 Review.
-
Efficacy of Conversion to Aflibercept for Diabetic Macular Edema Previously Refractory to Bevacizumab or Ranibizumab: A Meta-analysis of High-Quality Nonrandomized Studies.Ann Pharmacother. 2020 Aug;54(8):750-756. doi: 10.1177/1060028020904358. Epub 2020 Jan 31. Ann Pharmacother. 2020. PMID: 32005079
Cited by
-
Renal dysfunction associated with clinical response to intravitreal conbercept therapy for diabetic macular edema.Int J Ophthalmol. 2025 Mar 18;18(3):454-461. doi: 10.18240/ijo.2025.03.12. eCollection 2025. Int J Ophthalmol. 2025. PMID: 40103962 Free PMC article.
-
Evaluation of the Relationship Between Diabetic Macular Edema and Renal Function in a Latino Population.Ophthalmol Ther. 2023 Oct;12(5):2745-2755. doi: 10.1007/s40123-023-00787-w. Epub 2023 Aug 6. Ophthalmol Ther. 2023. PMID: 37543959 Free PMC article.
-
Acute Kidney Injury from Intravitreal Anti-vascular Endothelial Growth Factor Drugs: A Systematic Review and Meta-analysis of Randomized Controlled Trials.BioDrugs. 2023 Nov;37(6):843-854. doi: 10.1007/s40259-023-00621-6. Epub 2023 Sep 7. BioDrugs. 2023. PMID: 37676536
-
Recommendations for diabetic macular edema management by retina specialists and large language model-based artificial intelligence platforms.Int J Retina Vitreous. 2024 Feb 28;10(1):22. doi: 10.1186/s40942-024-00544-6. Int J Retina Vitreous. 2024. PMID: 38419083 Free PMC article.
References
-
- Massin P., Bandello F., Garweg J.G., Hansen L.L., Harding S.P., Larsen M., Mitchell P., Sharp D., Wolf-Schnurrbusch U.E., Gekkieva M., et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): A 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010;33:2399–2405. doi: 10.2337/dc10-0493. - DOI - PMC - PubMed
-
- Schmidt-Erfurth U., Garcia-Arumi J., Bandello F., Berg K., Chakravarthy U., Gerendas B.S., Jonas J., Larsen M., Tadayoni R., Loewenstein A. Guidelines for the Management of Diabetic Macular Edema by the European Society of Retina Specialists (EURETINA) Ophthalmologica. 2017;237:185–222. doi: 10.1159/000458539. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous