A Novel Method of Si and Si3N4 Powder Resources Recycling: Cold Bonding Briquettes
- PMID: 36013631
- PMCID: PMC9410509
- DOI: 10.3390/ma15165496
A Novel Method of Si and Si3N4 Powder Resources Recycling: Cold Bonding Briquettes
Abstract
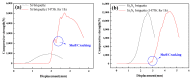
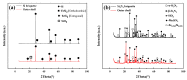
Silicon nitride (Si3N4) and silicon powder (Si) are two kinds of harmful solid waste in industrial production. As an environmental and low-consumption method, the cold-bonding technique is a novel method to utilize the problem of powder resource cycling. In this experiment, mechanical and high-temperature properties of Si and Si3N4 briquettes were studied after cold bonding. The results are as follows: (1) The compressive strength of the Si and Si3N4 briquettes increased with the improvement of molding pressure. With the same binder (1 wt.%) and water (10 wt.%) addition, the compressive strength of the Si3N4 briquette arrived at 12,023.53 N under 40 Mpa molding pressure, which is much higher than that of the Si briquette (942.40 N). The Si particles are uneven and irregular, which leads to an intense arch bridge effect in the Si briquette and the compressive strength decrease. Compared with Si powder, the particle size and shape of Si3N4 is small, uniform, and regular. The influence of the arch bridge effect is smaller than that in the Si briquette. (2) After being treated at 1473 K for 1 h, the compressive strength of the Si briquette increased to 5049.83 N, and the compressive strength of the Si3N4 briquette had a slight change. The surface of the briquettes was contacted with oxygen and reacted to form an outer shell which mainly contains SiO2 in the high-temperature treatment. FT-IR results have shown there were no extra impurities in cold-bonded briquettes when using the organic binder. (3) The microstructure of the cross section of the Si and Si3N4 briquettes after high-temperature treatment presented that oxygen entered the briquette through the pores and continued to react with the Si and Si3N4. The outer shell of the Si briquette grew and thickened continuously with the oxygen spreading in the Si briquette. However, because of the smaller particle size and regular shape, little oxygen diffused in the Si3N4 briquette. The outer shell of the Si3N4 briquette is fairly thin, so the compressive strength did not change too much.
Keywords: cold bonding; compressive strength; high-temperature properties; silicon; silicon nitride.
Conflict of interest statement
We declare that we have no financial or personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled.
Figures







References
-
- 04/00447 Manufacture of silicon nitride-iron powder for refractory materials: Ono, H. et al. Jpn. Kokai Tokkyo Koho JP 2003 119,081 (Cl. C04B35/626), 23 Apr 2003, Appl. 2001/318,988. Fuel Energy Abstr. 2004;45:52. doi: 10.1016/S0140-6701(04)91649-9. (In Japanese) - DOI
-
- Klemm H. Silicon Nitride for High-Temperature Applications. J. Am. Ceram. Soc. 2021;93:1501–1522. doi: 10.1111/j.1551-2916.2010.03839.x. - DOI
-
- Riley F.L. Silicon Nitride and Related Materials. J. Am. Ceram. Soc. 2000;83:245–265. doi: 10.1111/j.1151-2916.2000.tb01182.x. - DOI
-
- Bartnitskaya T.S., Vlasova M.V., Kakazei N.G., Tomila T.V. The role of silicon-particle defect structure during low-temperature nitriding. J. Mater. Sci. 1994;29:1701–1706. doi: 10.1007/BF00351284. - DOI
-
- Itoh T. Preparation of pure α-silicon nitride from silicon powder. J. Mater. Sci. Lett. 1991;10:19–20. doi: 10.1007/BF00724419. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

