Detoxification Response of Pseudomonas fluorescens MFAF76a to Gaseous Pollutants NO2 and NO
- PMID: 36013994
- PMCID: PMC9414441
- DOI: 10.3390/microorganisms10081576
Detoxification Response of Pseudomonas fluorescens MFAF76a to Gaseous Pollutants NO2 and NO
Abstract
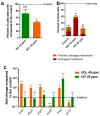
Bacteria are often exposed to nitrosative stress from their environment, from atmospheric pollution or from the defense mechanisms of other organisms. Reactive nitrogen species (RNS), which mediate nitrosative stress, are notably involved in the mammalian immune response through the production of nitric oxide (NO) by the inducible NO synthase iNOS. RNS are highly reactive and can alter various biomolecules such as lipids, proteins and DNA, making them toxic for biological organisms. Resistance to RNS is therefore important for the survival of bacteria in various environments, and notably to successfully infect their host. The fuel combustion processes used in industries and transports are responsible for the emission of important quantities of two major RNS, NO and the more toxic nitrogen dioxide (NO2). Human exposure to NO2 is notably linked to increases in lung infections. While the response of bacteria to NO in liquid medium is well-studied, few data are available on their exposure to gaseous NO and NO2. This study showed that NO2 is much more toxic than NO at similar concentrations for the airborne bacterial strain Pseudomonas fluorescens MFAF76a. The response to NO2 involves a wide array of effectors, while the response to NO seemingly focuses on the Hmp flavohemoprotein. Results showed that NO2 induces the production of other RNS, unlike NO, which could explain the differences between the effects of these two molecules.
Keywords: NO2; P. fluorescens; cell morphology; membrane; membrane integrity.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




References
-
- World Health Organization . WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide. World Health Organization; Geneva, Switzerland: 2021. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

