The Role of Thermokarst Lake Expansion in Altering the Microbial Community and Methane Cycling in Beiluhe Basin on Tibetan Plateau
- PMID: 36014037
- PMCID: PMC9412574
- DOI: 10.3390/microorganisms10081620
The Role of Thermokarst Lake Expansion in Altering the Microbial Community and Methane Cycling in Beiluhe Basin on Tibetan Plateau
Abstract
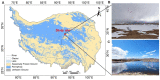
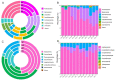
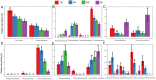
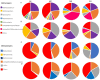
One of the most significant environmental changes across the Tibetan Plateau (TP) is the rapid lake expansion. The expansion of thermokarst lakes affects the global biogeochemical cycles and local climate regulation by rising levels, expanding area, and increasing water volumes. Meanwhile, microbial activity contributes greatly to the biogeochemical cycle of carbon in the thermokarst lakes, including organic matter decomposition, soil formation, and mineralization. However, the impact of lake expansion on distribution patterns of microbial communities and methane cycling, especially those of water and sediment under ice, remain unknown. This hinders our ability to assess the true impact of lake expansion on ecosystem services and our ability to accurately investigate greenhouse gas emissions and consumption in thermokarst lakes. Here, we explored the patterns of microorganisms and methane cycling by investigating sediment and water samples at an oriented direction of expansion occurred from four points under ice of a mature-developed thermokarst lake on TP. In addition, the methane concentration of each water layer was examined. Microbial diversity and network complexity were different in our shallow points (MS, SH) and deep points (CE, SH). There are differences of microbial community composition among four points, resulting in the decreased relative abundances of dominant phyla, such as Firmicutes in sediment, Proteobacteria in water, Thermoplasmatota in sediment and water, and increased relative abundance of Actinobacteriota with MS and SH points. Microbial community composition involved in methane cycling also shifted, such as increases in USCγ, Methylomonas, and Methylobacter, with higher relative abundance consistent with low dissolved methane concentration in MS and SH points. There was a strong correlation between changes in microbiota characteristics and changes in water and sediment environmental factors. Together, these results show that lake expansion has an important impact on microbial diversity and methane cycling.
Keywords: Tibetan Plateau; co-occurrence network; lake expansion; microbial community; sediment-water; thermokarst lake.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Diversity of primary vegetation species of lake shore impacts largely carbon emissions in thermokarst lakes on the Qinghai-Tibet plateau.Water Res. 2025 Mar 15;272:122946. doi: 10.1016/j.watres.2024.122946. Epub 2024 Dec 10. Water Res. 2025. PMID: 39674141
-
Methane emissions from thermokarst lakes must emphasize the ice-melting impact on the Tibetan Plateau.Nat Commun. 2025 Mar 11;16(1):2404. doi: 10.1038/s41467-025-57745-2. Nat Commun. 2025. PMID: 40064902 Free PMC article.
-
Distinct Microbial Assemblage Structure and Archaeal Diversity in Sediments of Arctic Thermokarst Lakes Differing in Methane Sources.Front Microbiol. 2018 Jun 7;9:1192. doi: 10.3389/fmicb.2018.01192. eCollection 2018. Front Microbiol. 2018. PMID: 29930542 Free PMC article.
-
Metagenomics Unveils Microbial Diversity and Their Biogeochemical Roles in Water and Sediment of Thermokarst Lakes in the Yellow River Source Area.Microb Ecol. 2023 Apr;85(3):904-915. doi: 10.1007/s00248-022-02053-1. Epub 2022 Jun 2. Microb Ecol. 2023. PMID: 35650293
-
Response of dissolved organic matter (DOM) and microbial community to submerged macrophytes restoration in lakes: A review.Environ Res. 2023 Aug 15;231(Pt 2):116185. doi: 10.1016/j.envres.2023.116185. Epub 2023 May 17. Environ Res. 2023. PMID: 37207736 Review.
Cited by
-
Oxygen-driven divergence of marine group II archaea reflected by transitions of superoxide dismutases.Microbiol Spectr. 2024 Jan 11;12(1):e0203323. doi: 10.1128/spectrum.02033-23. Epub 2023 Dec 4. Microbiol Spectr. 2024. PMID: 38047693 Free PMC article.
References
-
- Narancic B., Wolfe B.B., Pienitz R., Meyer H., Lamhonwah D. Landscape-gradient assessment of thermokarst lake hydrology using water isotope tracers. J. Hydrol. 2017;545:327–338. doi: 10.1016/j.jhydrol.2016.11.028. - DOI
-
- Mu C., WAbbott B., Norris A.J., Mu M. The status and stability of permafrost carbon on the Tibetan Plateau. Earth-Sci. Rev. 2020;211:103433. doi: 10.1016/j.earscirev.2020.103433. - DOI
-
- Takakura H., Iijima Y., Fedorov A., Tanaka T. Permafrost and Culture:Global Warming and the Republic of Sakha (Yakutia), Russian Federation. Center for Northeast Asian Studies; Sendai, Japan: 2021. pp. 45–47. Center for Northeast Asian Studies Report 26.
Grants and funding
LinkOut - more resources
Full Text Sources

