Anti- Toxoplasma gondii IgM Long Persistence: What Are the Underlying Mechanisms?
- PMID: 36014077
- PMCID: PMC9415799
- DOI: 10.3390/microorganisms10081659
Anti- Toxoplasma gondii IgM Long Persistence: What Are the Underlying Mechanisms?
Abstract
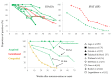
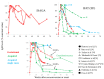
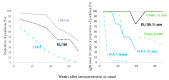
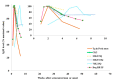
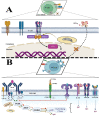
Diagnosis of Toxoplasma gondii acute infection was first attempted by detection of specific IgM antibodies, as for other infectious diseases. However, it was noted that this immunoglobulin declines slowly and may last for months or even years. Apart from the diagnostic problem imposed on clinical management, this phenomenon called our attention due to the underlying phenomena that may be causing it. We performed a systematic comparison of reports studying IgM antibody kinetics, and the data from the papers were used to construct comparative plots and other graph types. It became clear that this phenomenon is quite generalized, and it may also occur in animals. Moreover, this is not a technical issue, although some tests make more evident the prolonged IgM decay than others. We further investigated biological reasons for its occurrence, i.e., infection dynamics (micro-reactivation-encystment, reinfection and reactivation), parasite strain relevance, as well as host innate, natural B cell responses and Ig class-switch problems inflicted by the parasite. The outcomes of these inquiries are presented and discussed herein.
Keywords: IgM; Toxoplasma gondii; serological diagnosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Suzuki Y., Sa Q., Ochiai E., Mullins J., Yolken R., Halonen S.K. Toxoplasma gondii Model Apicomplexan Perspectives and Methods. 2nd ed. Elsevier Ltd.; Amsterdam, The Netherlands: 2014. Toxoplasma gondii; pp. 755–796. - DOI
Publication types
LinkOut - more resources
Full Text Sources

