A Portable 'Plug-and-Play' Fibre Optic Sensor for In-Situ Measurements of pH Values for Microfluidic Applications
- PMID: 36014146
- PMCID: PMC9416338
- DOI: 10.3390/mi13081224
A Portable 'Plug-and-Play' Fibre Optic Sensor for In-Situ Measurements of pH Values for Microfluidic Applications
Abstract
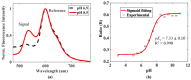
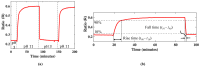
Microfluidics is used in many applications ranging from chemistry, medicine, biology and biomedical research, and the ability to measure pH values in-situ is an important parameter for creating and monitoring environments within a microfluidic chip for many such applications. We present a portable, optical fibre-based sensor for monitoring the pH based on the fluorescent intensity change of an acrylamidofluorescein dye, immobilized on the tip of a multimode optical fibre, and its performance is evaluated in-situ in a microfluidic channel. The sensor showed a sigmoid response over the pH range of 6.0-8.5, with a maximum sensitivity of 0.2/pH in the mid-range at pH 7.5. Following its evaluation, the sensor developed was used in a single microfluidic PDMS channel and its response was monitored for various flow rates within the channel. The results thus obtained showed that the sensor is sufficiently robust and well-suited to be used for measuring the pH value of the flowing liquid in the microchannel, allowing it to be used for a number of practical applications in 'lab-on-a-chip' applications where microfluidics are used. A key feature of the sensor is its simplicity and the ease of integrating the sensor with the microfluidic channel being probed.
Keywords: fluorescent sensor; microfluidics; optical fibre sensor; pH sensor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Werner J., Belz M., Klein K.F., Sun T., Grattan K.T.V. Fiber optic sensor designs and luminescence-based methods for the detection of oxygen and pH measurement. Measurement. 2021;178:109323. doi: 10.1016/j.measurement.2021.109323. - DOI
-
- Alam A.U., Qin Y., Nambiar S., Yeow J.T., Howlader M.M., Hu N.X., Deen M.J. Deen, Polymers and organic ma-terials-based pH sensors for healthcare applications. Prog. Mater. Sci. 2018;96:174–216. doi: 10.1016/j.pmatsci.2018.03.008. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

