Diagnosis Methods for COVID-19: A Systematic Review
- PMID: 36014271
- PMCID: PMC9415914
- DOI: 10.3390/mi13081349
Diagnosis Methods for COVID-19: A Systematic Review
Abstract
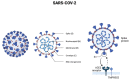
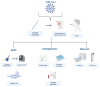
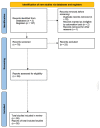
At the end of 2019, the coronavirus appeared and spread extremely rapidly, causing millions of infections and deaths worldwide, and becoming a global pandemic. For this reason, it became urgent and essential to find adequate tests for an accurate and fast diagnosis of this disease. In the present study, a systematic review was performed in order to provide an overview of the COVID-19 diagnosis methods and tests already available, as well as their evolution in recent months. For this purpose, the Science Direct, PubMed, and Scopus databases were used to collect the data and three authors independently screened the references, extracted the main information, and assessed the quality of the included studies. After the analysis of the collected data, 34 studies reporting new methods to diagnose COVID-19 were selected. Although RT-PCR is the gold-standard method for COVID-19 diagnosis, it cannot fulfill all the requirements of this pandemic, being limited by the need for highly specialized equipment and personnel to perform the assays, as well as the long time to get the test results. To fulfill the limitations of this method, other alternatives, including biological and imaging analysis methods, also became commonly reported. The comparison of the different diagnosis tests allowed to understand the importance and potential of combining different techniques, not only to improve diagnosis but also for a further understanding of the virus, the disease, and their implications in humans.
Keywords: COVID-19; PCR; SARS-CoV-2; diagnosis; image analysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- WHO. 2020. [(accessed on 17 December 2020)]. Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/2....
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous